Batyrbekov E.O., Umerzakova M.B., Zhubanov B.A.
Institute of Chemical Sciences, Almaty,
Kazakhstan
Release of Antibacterial Drugs from
Polyurethane Materials
Introduction. Polymeric
materials are frequently used in diagnostic and therapeutic procedures of
modern medicine as different kinds of prosthetic implants and catheters. Their
benefits for the patient are beyond doubt. On the other hand, special
complications associated with the foreign-body material have occurred. One of
the most frequent complications which is now considered as the major problem in
prosthetic medicine is the biomaterial-related infection [1]. In most cases the
antibacterial therapy alone can not cure the infection and the post-surgical
removal of the infected device or implant material becomes necessary. One of
approaches to prevent foreign body infection is the incorporation of
antibacterial agents into polymer then serving as drug delivery system [2].
They might either inhibit the bacterial adhesion or kill adherent bacteria due
to the continuous release of the drug resulting in a high local antibiotic
concentration in the vicinity of the
polymeric device or implant.
Polyurethanes are an important class of
polymers that have found many applications as biomaterials due to their
excellent mechanical properties and relatively good biocompatibility. Many
biomedical devices are made from segmented polyurethanes (SPU) such as vascular
prosthesis, catheters, blood pumps, heart valves and insulation for pacemakers,
etc. [3,4]. However, like many synthetic polymers, polyurethane devices are
susceptible to foreign body associated infection.
The purpose of the present
study was development of polyurethane-based biomaterials with infection
resistant properties. The incorporation of
antibacterial drugs like rifampicin and ciprofloxacin into segmented
polyurethanes by solvent cast technique
was described.. The drug release characteristics of such systems and
antibacterial effect of the antibiotic-loaded polymers were discussed.
Experimental. Polyurethane was synthesised by two-step polymerisation method using polypropylene glycol, toluene-2,4-diisocyanate and 1,4-butanediol as chain extender. Solutions of diisocyanate was placed in a four-necked flask equipped with a stirrer, a nitrogen inlet, an outlet and a thermometer and then the polyether diol solution containing 0,5 wt.% of catalyst was added slowly. The molar ratio of polyol and diisocyanate was 1:2,2. The reaction was carried out at 110-115oC for 2 h in the nitrogen flow. A prepolymer with isocyanate end groups was obtained where the percentage of isocyanate was determined by the dibutylamine titration method. Then the reaction mixture was cooled to room temperature and the chain extender was added slowly. The overall NCO : OH ratio was 1:1. The chain extension reaction was carried out until all NCO groups were reacted as confirmed by the disappearance of the IR absorption band at 2260 cm-1. The reaction mixture was placed in Teflon dish and dried under vacuum under at 50oC for at last 48 h until constant weight. By varying the ratio of components SPU containing different hard and soft segment contents were synthesised. Polymeric films containing antibacterial drugs were prepared by solvent cast technique. Briefly, SPU was dissolved in an appropriated solvent and various amounts of antibiotics added to the solution. After careful evaporation of the solvent at 50°C, the drug-loaded films were furthermore evaporated for 24 h at reduced pressure to remove solvent completely.
The release behaviour of drugs from polyurethanes
was examined by means of immersing the disc-shaped samples of 0,3-0,5 mm
thickness and 10,0 mm diameter in a Ringer-Lock solution at 37°C with constant
stirring. The amount of drug released was determined by UV-spectrometry by
measuring the absorption maximum characteristic for each drug. UV spectra were
recorded on a Jasco UV/VIS-7850 (Japan) spectrophotometer.
Results and Discussion. Polyurethanes with different content of hard and soft segments were synthesised by a two-step polymerization (Scheme). The polyether diols were first reacted with two equivalents of the diisocyanate. Subsequent chain extension was obtained by reaction with an equivalent amount of a butanediol. Antibacterial drugs rifampicin or ciproflaxacin was incorporated as solution into the polymeric matrix. The obtained SPU films were contained homogeneously dissolved antibiotics.
2
O=C=N-R-N=C=O + HO-R’-OH ®
OCN-R-NH-CO-O-R’-O-CO-NH-R-NCO
~~~
R*-NCO + H2O ® ~~~ R* -
NH2 +
CO2
~
R*-NH2 + OCN-R*~ ® ~R-
NH-CO-NH- R*~
R – tolylene-2,4-diisocyanate
R’ – [-CH(CH3)-CH2 –O-]n - polypropylene oxide
The revenant parameters of the drug contained SPU were following: average molecular weight 160,000-210,000; content of hard segments 14.2-46.4 %. Polymeric mechanical properties were changed progressively with increasing of drug loading. Previously, we reported drug release from polyurethane materials in which different drugs were incorporated into polymeric matrix in a dispersed form [5,6]. In this study the antibiotic release behaviour from SPU monolithic matrix into modelling biological media was analysed. The typical example of rifampicin release is presented at figure.
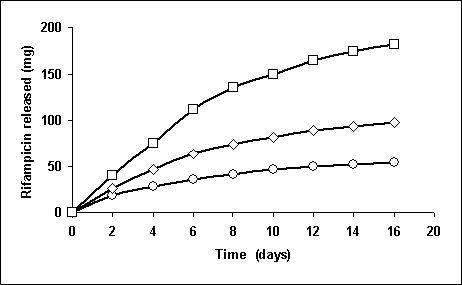
Fig. Release of
rifampicin from polyurethane films into Ringer-Lock
solution at 37oC.
Drug loadings (mg/g SPU): 100(O), 200(D), 300(ÿ)
All the release data show the typical pattern for a matrix controlled mechanism. The cumulative amount of drug released from the polyurethane was linearly related to the square root of the time and the release rate decreased with time. The process is controlled by the dissolution of the drug by its diffusion through the polymer in accordance with Fick law. The total amount of rifampicin is released in 30-34 days and ciprofloxacin in 28-30 days.
The antibacterial activity of drug released from the SPU was
determined by diffusion method against five different strain of bacteria. It
has been found that antibacterial drug incorporated into a polymeric matrix
have zone of inhibition on the level of free drug. Moreover, the
rifampicin-loaded samples exhibited high inhibitory response against a museum
strain of Mycobacterium tuberculosis.
Conclusion. In this study, the incorporation of antibiotics rifampicin and ciproflaxacin into segmented polyurethanes to obtain drug delivery devices was described. Antibiotic-loaded polyurethane films show a high initial release rate and the matrix-controlled release for more 20 days. The release characteristics depends on drug loading and polyurethane macrodomain structure. The antibiotic-loaded polyurethane systems might be useful for prevent foreign body infection.
References
1.
Sugarman B.,Young
E.J. (eds). Infection associated with prosthetic devices, CRC Press, Boca
Raton, FL.1984.
2.
Schierholz J.,
Jansen B., Jaenicke L., Pulverer G. Biomaterials.1994, 15, 996-999
3.
Lelah M.D., Cooper
S.L. Polyurethanes in Medicine, CRC Press, Boca Raton, FL, 1986.
4.
Plank H., Syre.I.,
Egbers G. (eds), Polyurethanes in Biomedical Engineering: Progress in
Biomedical Engineering, Elsevier, Amsterdam.1987.
5. Iskakov R.,
Batyrbekov E., Leonova M., Zhubanov B. Journ Appl Polym Sci. 2000, 75, 35-41.
6.
Batyrbekov E.,
Rukhina L., Zhubanov B., Smailova G. Polym Int. 1997, 43, 317-420.