PhD Pavlova A. P.1,
Doctor of Chemical Sciences Mittova I. Ya.1,
PhD Mittova V. O.2,
PhD student Dinh Van Tac1.
1 Voronezh State University
2 Voronezh State Medical Academy named after N.N. Burdenko
The properties of nanocrystals
Y3Al5O12,
Y3Fe5O12, Y3-xLaxFe5O12
garnet structure, synthesized by chemical precipitation
method.
Nanocrystalline garnets Y3Al5O12 (YAG), Y3Fe5O12, (YIG) and solid solutions based
on them attract great interest due to their optical properties. Iron garnets are under scrutiny because of their magneto-optical properties and prospects for practical use as functional materials
in microwave engineering, recording and storing information, nanocomposites,
and various magneto-optical devices [1, 2]. The crystals of yttrium aluminum garnet due to their
relatively stable lattice structure and high thermal conductivity are used as laser materials and coatings
for electronic
devices.
Out of the possible chemical methods of obtaining these
nanomaterials , the sol-gel method is very promising since it allows
to reduce the duration of sintering, decrease the
temperature of synthesis and to provide better control of the
size of the particles obtained.
Developed processes for the synthesis of nanopowders Y3Al5O12,
Y3Fe5O12, Y3-xLaxFe5O12 include the following steps: deposition of the metal hydroxides, Fe (III), Y, Al, La in water by the method of [1, 3, 4], filtration, drying, their subsequent firing in air to complete the dehydration, and the formation of the final product.
The method consists of obtaining Y3Fe5O12 by preparing an aqueous solution containing 0.008 M Fe(NO3)3, 0.008× 3/5 M YCl3, boil it for 5 minutes and then cool it to room temperature. Then into the resulting solution was poured drop by drop with constant stirring with a mechanical stirrer (speed of 3000 rev/min) 0.3 M aqueous solution of ammonia in an amount necessary for complete precipitation of the cations Y3+
and Fe3+. After the introduction of ammonia, stirring was continued for another 15 min. Then the precipitation was filtered, washed and dried at room temperature until its weight becomes constant. Nanocrystals are Y3Fe5O12 calcination of the precipitate in a muffle furnace at 1000 °C for 4 h. The process of doping Y3-xLaxFe5O12 performed on the same technology, taking into account the values of x = 0.2, 0.4, 0.6 [1, 3].
For the synthesis of liquid-phase samples Y3Al5O12 the method of using aqueous solutions of
nitrates Al, Y (0.15 M and 0.15 M, respectively) and 1.5 M solution
of NH4HCO3 as precipitator. The speed of adding of salt
solution to the precipitant in the magnetic stirrer 3
ml/min at room temperature, processing time - 21 minutes. After
aging the suspension (1 h) and filtration, the precipitate
is dried in the air 24 hours, the regime of calcination - 955
°C for 1 hour.
Phase composition of samples determined by means of X-ray diffraction (XRD, DRON-4 diffractometer, СоКа - radiation, λ =
0,17902 nm) with the calculation
of the
size of
coherent scattering regions (CSR) by Scherrer for the determination of particle size. Alternative methods for the
determination of particle size - dynamic and static light scattering (spectrometer Photocor Complex) [1, 3] and high-voltage transmission electron microscopy (TEM, PC-100 BR, fig.). The results of the calculations the average particle size obtained from the three methods used are summarized in the table.
The magnetic properties of nanocrystals Y3-xLaxFe5O12 tested for vibration magnetometer at room temperature [1, 3]. It is shown that with increasing lanthanum content of the saturation magnetization first increases and then decreases, the maximum corresponds to x = 0.2 (Y2.8La0.2Fe5O12) and amounts to 28,123 Am2/kg. For samples Y2.6La0.4Fe5O12
(x = 0.4) and Y2.4La0.6Fe5O12
(x = 0.6) of
the saturation magnetization are respectively 25,747 and 24,758 Am2/kg, which is lower than that of the sample in the absence of dopant Y3Fe5O12 (26.141 Am2/kg).
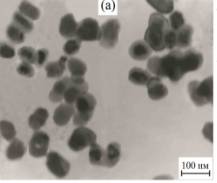
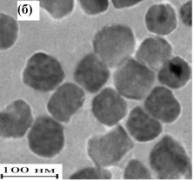
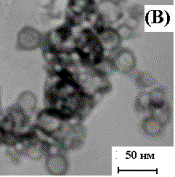
Fig. TEM images of nanopowders Y3-xLaxFe5O12
(a), Y3Fe5O12 (b), Y3Al5O12 (c)
Table. The average diameter of the obtained nanocrystals.
|
|
XRD |
dynamic and static light scattering |
TEM |
|
Y3Fe5O12 |
54 nm |
57 nm |
68 nm |
|
Y3Al5O12 |
26 nm |
40 nm |
35 nm |
|
Y3-xLaxFe5O12 х = 0 – 0.6 |
54 nm – 44 nm |
57 nm – 49 nm |
for x= 0.6 65 nm |
From the data of XRD and LRSMA (local X-ray microanalysis in the electron microscope JEOL-JSM-6380LV), it follows that the substitution of yttrium,
lanthanum, limiting the formation of solid solutions of Y3-xLaxFe5O12
up to 0.8. For x = 0.8 phase appears LaFeO3, consequently, for x ≥ 0.8 the region of homogeneity breaks down and released the second phase LaFeO3. With increasing content of lanthanumin the solid solutions Y3-xLaxFe5O12
decreases the size of the particles and the cell parameters increase. When x varies from about 0.6 to the cell parameter (a) increased from 12,328 to 12.421 Å, which is associated with an increase in the ionic radius of La3+ compared to Y3+.
References:
1. Dinh Van Tac, Mittova V. O., Almyasheva O. V., Mittova I. Ya. Synthesis,
structure and magnetic properties of nanocrystalline Y3-xLaxFe5O12 (0 ≤ x ≤ 0.6) // Inorg. materials. 2012. V. 48.
№ 1. Р. 81–86.
2. Eliseev А. А, Lukashin A.
V. Functional Nanomaterials. M.: FIZMATLIT, 2010. P. 456.
3. Dinh Van Tac, Mittova V. O., Mittova I. Ya. Effect of lanthanum content
and annealing temperature on the size and magnetic properties Y1-xLaxFeO3, obtained
by the sol-gel method // Inorg. materials. 2011. V. 47. № 5. P. 590-595.
4. Ji-Guang Li
et al. Co-precipitation synthesis and sintering of yttrium aluminum garnet
(YAG) powders: the effect of precipitant // Journal of the European Ceramic
Society. 2000. № 20. P. 2395-2405.