1Kushkarina S.M., 2Shaimardan M.
1A.B.Bekturov Institute of chemical sciences Almaty,
Kazakhstan
2Kazakh- British Technical
University, Almaty, Kazakhstan
The effect of promoter and activated
carbon carriers on rhodium catalyst in the process of hydrogenation of benzene
Development of new
active and selective catalysts of removing a small amount of benzene from
gasoline is an issue of current importance, as during incomplete combustion of
benzene containing gasoline in exhaust gases very strong carcinogen, namely
benzopyrene is formed. According to existing Euro-2 standards the amounts of
benzene in gasoline must not exceed 5% and Euro-3 restricts the amounts of
benzene up to 1%. The most effective way of removing benzene from gasoline is
its catalytic hydrogenation into more ecologically safe cyclohexane. The
present report discusses rhodium catalyst supported on sibunit (Siberian
activated carbon) modified with molybdenum salt and its influence on the
reaction of hydrogenation of benzene and toluene. In the presence of rhodium
catalyst [2] the factors, like reaction medium, type of carrier, temperature,
and hydrogen pressure can greatly effect on the process of hydrogenation of
benzene . In addition to these, modification of catalyst can change the
selectivity and activity of benzene hydrogenation.
Rhodium is the most active catalyst for
hydrogenation of benzene [1, 2]. It has been observed that modifier reduces the
reaction rate of hydrogenation of benzene and has no effect on the rate of
hydrogenation of toluene. 3%Rh/sibunit catalyst were investigated by scanning
electron microscopy. The effect of molybdenum on the catalyst surface has been
discussed and compared with experimental data.
3%Rh/sibunit catalyst
has been prepared by impregnation method and modified with ammonium molybdate,
further 3%Rh/sibunit+0,03%Mo has been used for the reaction of hydrogenation of
benzene and toluene. Activated carbon carrier was heated in quartz furnace during
four hours in nitrogen atmosphere at the temperature of 400°C. Modification of
catalyst was also prepared by impregnation. Hydrogenation of benzene and
toluene has been carried out in the kinetic hydrogenation autoclave at the
temperature of 40°C and hydrogen pressure 40atm.
Early it has been
determined that the rate of hydrogenation of xylene and cumene is lower than
that of benzene and toluene [2]. That is why in this experiment the selectivity
of benzene hydrogenation has been compared to the selectivity of toluene. Picture
1 shows hydrogenation rates of benzene and toluene catalyzed by modified and
non- modified catalysts.
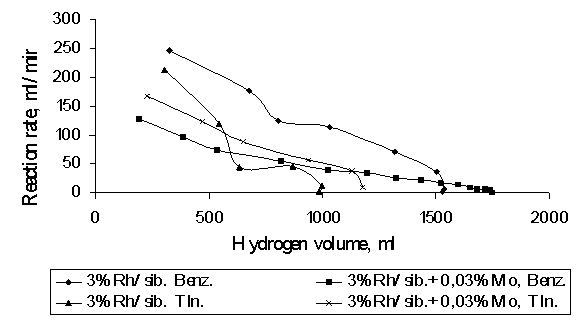
Picture 1. Hydrogenation
of benzene and toluene on 3%Rh/sibunit and 3%Rh/sibunit+0,03%Mo catalysts.
It
can be seen that initial hydrogenation rate of benzene on 3%Rh/sibunit catalyst
is 110ml/min and 3%Rh/sibunit+0,03%Mo is lower, it is 70ml/min. And
hydrogenation rate of toluene on 3%Rh/sibunit and 3%Rh/sibunit+0.03%Mo is
almost the same. In early publications [3] it has been observed that molybdenum
salts block the surface of rhodium and activated carbon, so it will cause
resistance for adsorption of hydrogen. The lower hydrogenation rate of benzene
on modified catalyst can be explained with the same reason.
Picture 2 illustrates how rhodium salt
has been equally covered on a surface of catalyst. After modification of
catalyst the elements distribution on the catalyst surface is different.
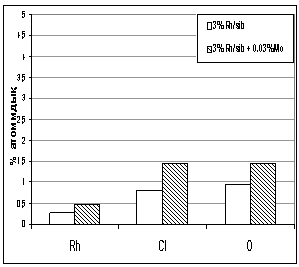
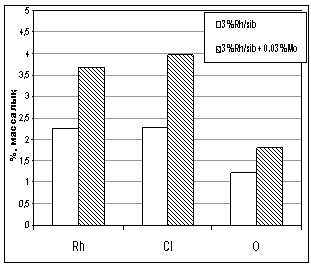
Picture 3. Elements assignment in atomic and
mass percentage.
As sibunit is graphite structured activated
carbon, its pour size is almost the same, so molybdenum oxide covers surface
area of rhodium and activated carbon thereby worsening catalyst activity.
Compared to fruit- stone activated carbon that possesses with different pore
size, molybdenum oxides cover only the biggest pore and the smallest pore can
be adsorbed by benzene.
Reference
1.
Ñîêîëüñêèé Ä.Â. Ãèäðèðîâàíèå
â ðàñòâîðàõ. ÊàçÑÑÐ. – Àëìàòû: Íàóêà, 1979, 364ñ.
2. Êîíóñïàåâ Ñ.Ð. Øàéìàðäàí Ì. // Õèì. æóðíàë Êàçàõñòàíà, 2006, ¹ 1. Ñ.154-174.
3. Reyes P., Fernández J., Concha I., Pecchi G., Granados M. L., Fierro
J. L. G. // Catal. Lett. 1995. – 34, ¹3 – 4. C.331 – 341.