Медицина/6.Экспериментальная и клиническая
фармакология
Babiy O.P., Grehirchak N.M.1, Shpak
E.G.2
1National University
of Food Technologies, Ukraine
2R.Y. Kavetsky Institute of Experimental Pathology, Oncology and
Radiobiology NAS of Ukraine, Kyiv
SCREENING ADJUVANTS ORGANIC AND INORGANIC NATURE FOR CONSTRUCTING CANCER VACCINES
Problem of tumor growth is the most important in
modern medicine and biology. Above it are dealt hundreds of medical
laboratories around the world. In this area has been done much research, but
its solution is still far ahead. Therefore, the search for new drugs to treat
cancer is an open topic. At present the views of scientists around the world
aimed at using alternative means, namely the methods of biotherapy, especially
immunotherapy of cancer patients [1]. The last has several areas, including the
use of cytokines, monoclonal antibodies and specific anti-tumor vaccines. The use of anticancer vaccines
is very promising approach,
it’s made on the tumor associated antigens, which are based on the formation of specific reactions of
antitumor immunity. It should be noted that most of the tumor associated antigens have low immunogenicity, which leads to the need to find different ways to improve the efficiency of anticancer vaccines [2]. The one of ways to enhance the immune response to this antigens is using of adjuvants, which spectrum is quite broad. However, the impact of adjuvant on immunogenicity
of tumor associated
antigens and the dynamics
of nonspecific and specific
antitumor responses remains
is still unspecified.
The
work was aimed on the selection of potential adjuvant for designing antitumor vaccines and study their
effects on the immune system in animal
experiments
with Lewis lung carcinoma
(LLC).
In experiment
were used male Balb / c line 2-2.5 months
old and average weight 18
- 20 g mice obtained from vivarium of R.E. Kavetsky Institute
of Experimental Pathology, Oncology and Radiobiology NAS of Ukraine. As a model of tumor growth
was used the Lewis lungs metastatic epidermoid carcinoma.
A
series of experiments, namely triple immunization
of animals by chicken embryonic proteins (0.1
mg of protein per injection) were
carried out in mono or in combination with adjuvant:
lipids from cell
B. subtilis B-7025 molecular weight 18.5 kDa and 70 kDa (0.006 mg/injections),
microbial cell BCG
(0,3×108 CFC/injections),
colloidal silver (Ag) and suspension of iron oxide (Fe3O4) in 2% solution
of polidekstran (0.06 mg/injections). For intact control (IC) were used animals
injected with NaCl.
Immunological examination included:
determination of cytotoxic activity and
antibody-dependent cytotoxic activity of lymphocytes and macrophages, cooperative
cytotoxic activity of effecter cells, cooperative antibody-depended cellular
cytotoxicity of lymphocytes and macrophages, ELISA detection
of generated antibodies specific to
chicken embryonic proteins or tumor antigens
LLC.
As the results of investigations
evidenced, the introduction of chicken embryonic proteins by
themsalve independently, so do in combining with adjuvant caused inhibition
of growth of LLC in experiments
on animals. Stability of this effect remains at
all stages of growth of
experimental tumors. The
comparative analysis of the size of
primary tumors in animals from different groups at
the end of the experiment (34th day) showed that in
animals, who received
the vaccine based on CEB with glycoproteins
B. subtilis B-7025 tumor volume
was 13% lower
than in the IC . It is necessary to note that the degree of inhibition of
tumor growth in terms of different tumor process was uneven.
The
dynamic of growth of
the LLC after interruption to the animals studied vaccines
was different. In the primary stages
of tumor process the interruption of all
the studied substances resulted inhibition of tumor growth. Through the development of the tumor suppressive effect of the
studied preparations has been gradually
decreased. The most expressive effect was in
animals that received the vaccine based on CEP and glycoproteins as adjuvant.
Within the immunological experiments
there were established that the maximal
synthesis of antibodies was observed in groups of animals, which
as an adjuvant to
CEP got metabolite B. subtilis B-7025 with mol.
weight 18.5 kDa and
70 kDa and peptidoglycan
of
S.aureus cells. In the group of
animals where as adjuvant were
used BCG synthesis
of antibodies was lower, than in
group with chicken embryonic proteins
(Fig. 1). According to this we
can conclude that BCG activates the cellular
immunity and suppress of humoral.
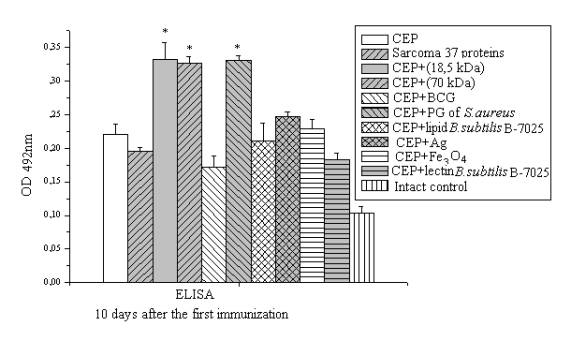
Figure 1. ELISA detection of serum in experimental groups
specific to chicken embryonic proteins provided
immunization CEP with adjuvants.
Similar
results were also
obtained in assessing the accumulation of antibodies to proteins of Sarcoma 37
(Fig. 2).
It was found that in all experimental groups, the
level of medium molecular
circulating immune complexes (CIC) in serum was higher compared with intact animals. In
mice immunized with
CEP and Fe3O4,
CIC level exceeded
the same index in animals that received no adjuvant
chicken embryonic proteins. Add to
CEP almost all
investigated adjuvant (except a mixture of lipids B. subtilis B-7025) led to a
decrease in titer of antibodies
against protein S-37.
As a result of the test to determine cytotoxic activity
of lymphocytes against cells S-37, demonstrates
that the introduction of CEP
is not likely led
to its change.
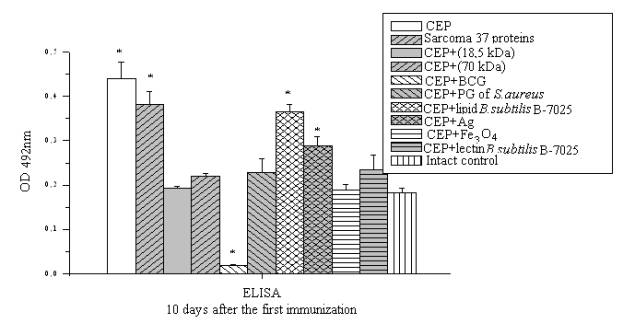 Figure 2.
ELISA detection of serum proteins in experimental groups against Sarcoma
37.
Figure 2.
ELISA detection of serum proteins in experimental groups against Sarcoma
37.
It is shown that
a mixture of lipids B. subtilis B-7025 has immunotoxic
effects on the mice Balb/c and does not
cause inflammatory reactions.
Introduction of CEP with adjuvants, mainly with
lipids of B. subtilis B-7025, induces the
formation of specific IgG in the
serum of animals. These data
suggest the feasibility study
of lipids as potential immunomodulating agents for their further
use in oncology practice.
REFERENCES:
1. Aucouturier J. The use of oil adjuvants
in therapeutic vaccines / / Vaccine. - 2006. - № 24. - P. 2 - 45.
2. Mesa C.,Fernandez L.E. Challenges facing
adjuvants for cancer immunotherapy / / Immunol. Cell. Biol. - 2004. - V. 82, №
6. -P. 644-650.
3. Herlyn D. Advances in cancer vaccine
development / / Ann. Med. - 1999. - № 31. -P. 66 - 78.
4. Cox J.C. Adjuvants - a classification and
review of their modes of action / / Vaccine. - 1997. - № 15. - P. 248 - 256.