Thermodynamico-kinetic equation of the
universe
Dr. Ivan
M.Kolesnikov,Dr/ Kolesnikov S.I., Vinokurov V.A.
Gubkin Russian State University of Oil
and Gas, Moscow, Leninsky prospectus 65, Tel/Fax:8-499-135-87-25
This article presents a new
approach to the description of the thermodynamics of equilibrium-none-quilibrium
processes in the annex to the universe. The equation takes into account
spontaneously and non-spontaneously occurring processes, the equations for the
flow of functions and functionals.
The universe may be
presented as extended in space and time material objective reality. It is
filled with phase-open systems. Between these systems occurs exchange of energy
and matter through occurring simultaneously, continuously and interconnected
equilibrium-none-quilibrium processes. This
approach to development of the equation the universe was not addressed properly
in scientific studies of classic thermodynamics of equilibrium-none-quilibrium
processes.
One of basic principles as
respects to the universe states that overall reserve of internal energy in the
universe is constant. Redistribution of energy between objects in the universe
may be connected to the heat and the work, according to the first law of
thermodynamics, videlicet:
![]() (1)
(1)
![]() (2)
(2)
Based on classical
thermodynamics, these equations determine that in the universe heat of
non-spontaneously occurring processes increases the supply of internal energy
for the phase-open system. Unfortunately in these equations don’t take into
account type of the process in the phase-open system. But it cannot be
generalized for the universe, because in accordance to stated above principle
in the universe we have the equality:
![]() (3)
(3)
The second concept for
creation the equation of the universe came to be the new thermodynamic
function, introduced in thermodynamics as the entropy by Rudolf Clausius.
Entropy is a thermodynamic function that interconnects the structure in phase-open
system and possible direction of occurring processes. Clausius defined the
Principle of existence of entropy of the concept of increasing entropy,
analytically expressed in the following form:
![]() (4)
(4)
![]() (5)
(5)
Analytical expression of the
concept of existence of entropy reflects the following principles:
-
The system is finde not far from
the equilibrium state.
-
The system transgresses to the equilibrium by the equilibrium way (*
this state is a science fiction)
-
A plus sign before a sign of entropy change defines the spontaneously
occurring process.
The Ðrinciple of
increasing entropy, defined for isolated
systems, reflects capability of exclusively spontaneously
occurring system state changes, under constant volume and intraatomic energy of
the system. This assumption is also a science fiction because an isolated
system does not interact with the universe.
During system transition to
equilibrium state with U,v=const energy change vanishing due to following
criteria:
![]() (6)
(6)
With this equation Clausius
hypothesized the heat death of the universe. However, assumption (5) was
defined for isolated system and cannot be applied to the open system, such as
the universe, without additional criteria. It can only be extended to the
universe based on the fourth law of thermodynamics( I.M.Kolesnikov), which will
be formulated later. Deficiencies of criterion
(6) in the annex to the universe are as follows:
-
It does not take into account the type of occurring process, with which
the system transcends to the equilibrium state.
-
Constancy of U and V is inapplicable to the universe since it
determines finiteness of their values in conditions of the unlimited universe.
-
The fact that this criterion can only be used for locally isolated
system is not accounted for.
According to Clausius both criteria (5) and (6) is supplement each other:
![]() (7)
(7)
![]() (8)
(8)
These criteria do not fully
reflect the behavior of the local phase-open systems
interacting with the environment.An isolated
system is a science fiction but only for isolated systems the total change in
entropy leads to its increase to achieve the equilibrium.One may not extend
conclusions obtained for isolated systems onto the universe since in the
universe spontaneous and –non-spontaneous processes occurring simultaneously,
continuously and interconnected, which was not taken into account by Clausius.
According to Clausius, only spontaneous occurring in the universe [1], which is
absurd. As criterion allowing to determine the direction of occurring processes
for the universe may be used the following expression:
![]() (9)
(9)
Where the signs:
>defines the concept of
increasing entropy of Clausius
= the concept of existence
of the entropy of Clausius,
<the concept of
decreasing entropy of Kolesnikov.
During the development of
thermodynamics of irreversible processes in the middle of the twentieth
century, N.Belokon [2], implicitly shared heat and entropy into two types:
-
Q¹,S¹ - the processes occurring within the system
-
Q¹¹, S¹¹- heat-exchange and entropy to the
environment.
N.Belokonexpressedthe
heat and entropy change in the following form:
δQ=δQ¹+δQ¹¹,
dS=dS1+dS11, δW=δW1+δW11 (10)
The equation of the first law of thermodynamics is:
δQ =dU+δW (11)
Accounting expressions (10)it
can be represented in a more specific form, although not entirely adequate for
real systems:
δQ¹+δQ¹¹=dU+δW1+δW11 (12)
And the fundamental equation
of thermodynamics will take the following form:
dS1+dS11= dU+δW1+δW11 (13)
These equations do not
reflect types of occurring processes (spontaneous and non-spontaneous) nor
division of internal energy. Thoughthey take into account interaction of the
system with the environment but not the loss, they already partially reflect the
thermodynamics of irreversible processes.
At the same time in Belgium
Ilya Prigoginecommittedly begins to develop thermodynamics of irreversible
spontaneously occurring processes, [3] excluding thermodynamics of
non-spontaneously occurring processes.In his works, he also identifies internal
processes and the processes occurring in the environment by using the following
expressions:
δQ=δiQ+δeQ, dS=diS+deS (14)
Where δiQ, diS – flow of heat and
entropy, due to the processes occurring inside the system. And δeQ – flow of heat and entropy, due to the
interaction with the environment.
We may note that Prigogine’s statement, that
entropy difference diS,due to changes within the system, never has
a negative value[3, 4, 5, 6], is only valid for isolated systems and may have a
negative value for open systems due to external influence.
According to Prigogine the fundamental equation of
thermodynamics will take the following form:
diS+deS=dU+δiW+δeW (15)
This equation is set for a single phase interacting
with the environment.
Let the interacting system have two phases (1 and 11).
Then for these phases heat exchange and entropy change may be presented by the
following equation:
δ1Q=δ1iQ+δ1eQ, δ11Q=δ11iQ+δ11eQ (16)
Dividing by
corresponding temperatures derives following expressions:
δ1Q/Ò=δ1iQ/Ò1+δ1eQ/Ò1, δ11Q/Ò=δ11iQ/Ò11+δ11eQ/Ò11 (17)
Considering the
concept of entropy, these equations convers to the following form:
dS=d1S+d11S (18)
Substitute (17) to
(18) and get following equation:
dS=δ1iQ/Ò1+δ1eQ/Ò1+δ11iQ/Ò11+δ11eQ/Ò11 (19)
Merge terms with
the same subscript:
dS=δ1iQ/Ò1+δ11iQ/Ò11+δ1eQ/Ò1+δ1eQ/Ò1 (20)
Or
dS=di1S+di11S+de1S+de11S (21)
This equation
reflects only thermodynamic processes but not chemical and other processes.
We already can express the fundamental equation of
thermodynamics for two-phase subsystem within the system which interacts with
the environment.
T1edi1S+Ti11di11S+Te1de1S+Te11de11S=dU+δi1W+δe1W+δi11W+δe11W (22)
But it still does not reflect types of occurring
processesnor division of internal energy and the loss. The equation can be transformed
furthermore based on the forth law of thermodynamics, which reflects the new
thermodynamics of equilibrium-nonequilibrium processes.
The forth law of thermodynamics was enunciated in the
following form [7,8]: Spontaneous and nonspontaneous processes occurring simultaneously,
continuously and interconnected
in phase-open systems and the environment.Moreover,
spontaneously occurring processes pass with increasing entropy change
(pos-entropy) and decreasing of free internal energy, and non-spontaneously
occurring processes pass with increasing free internal energy’s reserve and
decreasing entropy change (neg-entropy).
For a single phase-open
system the fundamental equation of thermodynamics will take the following form:
Ti1di1SSOP+Ti1di1SNSOP+Te1de1SSOP+Te1de1SNSOP=diUSOP+diUNSOP+deUSOP+deUNSOP+δi1WSOP+δi1WNSOP+δe1WSOP+δe1WNSOP (22)
This expression
still does not take into account the loss in each occasion of entropy change,
internal energy change and work change. Supplementing the loss to each term of
(22) we will get the following expression
Ti1di1SSOP+(Ti1di1SSOP)loss+Ti1di1SNSOP+(Ti1di1SNSOP)loss+Te1de1SSOP+(Te1de1SSOP)loss+Te1de1SNSOP+(Te1de1SNSOP)loss=diUSOP+(diUSOP)loss+diUNSOP+(diUNSOP)loss+deUSOP+(deUSOP))loss+deUNSOP+δi1WSOP+(δi1WSOP)loss+δi1WNSOP+(δi1WNSOP)loss+δe1WSOP+(δe1WSOP)loss+δe1WNSOP+(δe1WNSOP)loss (23)
This equation determines the stationary state of the subsystem of a single
phase-open system which interacts with the environment. It can be converted to
the form applicable to the universe by summation, within its boundary, from 1 to ∞. As a result, receiving following expression for non-flow systems:
![]() {TijSSOP+(TijdijSSOP)loss+TIjdijSNSOP+(TijdijSNSOP)loss+TejdejSSOP(TjedejSSOP)loss+TejdejSNSOP+(TejdejSNSOP)loss}=
{TijSSOP+(TijdijSSOP)loss+TIjdijSNSOP+(TijdijSNSOP)loss+TejdejSSOP(TjedejSSOP)loss+TejdejSNSOP+(TejdejSNSOP)loss}=
![]() {dijUSOP+(dijUSOP)loss+dijUNSOP+(dijUNSOP)loss+dejUSOP+(dejUSOP)loss+dejUNSOP+(dejUNSOP)NSOP}+
{dijUSOP+(dijUSOP)loss+dijUNSOP+(dijUNSOP)loss+dejUSOP+(dejUSOP)loss+dejUNSOP+(dejUNSOP)NSOP}+
![]() {δejWSOP+(δijWSOP)loss+δijWNSOP+(δijWNSOP)loss+δejWSOP+(δejWSOP)loss+δejW NSOP+(δejWNSOP)loss} (24)
{δejWSOP+(δijWSOP)loss+δijWNSOP+(δijWNSOP)loss+δejWSOP+(δejWSOP)loss+δejW NSOP+(δejWNSOP)loss} (24)
Numerical value of the
sum varieties form j=1 to j=∞. Though, this form does not take into account
the energy flow and the functional flow. To allow this for the flows in the
universe, multiplying each part of the equation by dρ,dv,dτ
(parameters thatdetermine the density, linear velocity and time). The resulting
expression is the equation of the universe that includes the mass flow and
energy flow:
![]() {TijSSOP+(TijdijSSOP)loss+TIjdijSNSOP+(TijdijSNSOP)loss+TejdejSSOP(TejdejSSOP)loss+TejdejSNSOP+(TejdejSNSOP)loss}dρ,dv,dτ=
{TijSSOP+(TijdijSSOP)loss+TIjdijSNSOP+(TijdijSNSOP)loss+TejdejSSOP(TejdejSSOP)loss+TejdejSNSOP+(TejdejSNSOP)loss}dρ,dv,dτ=
![]() {dijUSOP+(dijUSOP)loss+dijUNSOP+(dijUNSOP)loss+dejUSOP+(dejUSOP)loss+dejUNSOP+(dejUNSOP)NSOP}dρ,dv,dτ +
{dijUSOP+(dijUSOP)loss+dijUNSOP+(dijUNSOP)loss+dejUSOP+(dejUSOP)loss+dejUNSOP+(dejUNSOP)NSOP}dρ,dv,dτ +
![]() {δejWSOP+(δijWSOP)loss+δijWNSOP+(δijWNSOP)loss+δejWSOP+(δejWSOP)loss+δejW NSOP+(δejWNSOP)loss}dρ,dv,dτ (25)
{δejWSOP+(δijWSOP)loss+δijWNSOP+(δijWNSOP)loss+δejWSOP+(δejWSOP)loss+δejW NSOP+(δejWNSOP)loss}dρ,dv,dτ (25)
According to
Prigogine thermodynamic functions and functional change in time, and
considering the concepts of entropy and fluctuations of thermodynamic functions
can be expressed as follows:
![]()
![]() etc. (26)
etc. (26)
Analytical equation of
the forth law of thermodynamics for non-stationary processes can be expressed
in the following form:
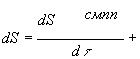
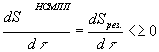 (27)
(27)
Where:
dSñìïï=diSñìïï+deSñìïïand
dSíñìïï=diSíñìïï+deSíñìïï (28)
It
was previously assumed that only one-way spontaneous processes may occur in the
system and the universe, but now we can see that above equations represents
two-way processes. Spontaneously occurring process may dominate over
non-spontaneously occurring process and vice versa. This statement is confirmed
by definition of the forth law of thermodynamics and substantively occurring
processes in phase-open systems and the universe. Spontaneously occurring
processes lead to degradation of the system while non-spontaneously occurring
processes result in its evolution.
Expression
(25) defines thechanges within the system, and in the local
environment, occurring over time dτ.
To determine the changes in the system and the universe over a single unit of
time the equation (25) differentiated with respect to time. The resulting form
is the general equation of the universe.
![]()
![]() {TijSSOP+(TijdijSSOP)loss+TIjdijSNSOP+(TijdijSNSOP)loss+TejdejSSOP(TejdejSSOP)loss+TejdejSNSOP+(TejdejSNSOP)loss}dρ,dv,dτ=
{TijSSOP+(TijdijSSOP)loss+TIjdijSNSOP+(TijdijSNSOP)loss+TejdejSSOP(TejdejSSOP)loss+TejdejSNSOP+(TejdejSNSOP)loss}dρ,dv,dτ=
![]()
![]() {dijUSOP+(dijUSOP)loss+dijUNSOP+(dijUNSOP)loss+dejUSOP+(dejUSOP)loss+dejUNSOP+(dejUNSOP)NSOP}dρ,dv,dτ +
{dijUSOP+(dijUSOP)loss+dijUNSOP+(dijUNSOP)loss+dejUSOP+(dejUSOP)loss+dejUNSOP+(dejUNSOP)NSOP}dρ,dv,dτ +
![]()
![]() {δejWSOP+(δijWSOP)loss+δijWNSOP+(δijWNSOP)loss+δejWSOP+(δejWSOP)loss+δejW NSOP+(δejWNSOP)loss}dρ,dv,dτ (29)
{δejWSOP+(δijWSOP)loss+δijWNSOP+(δijWNSOP)loss+δejWSOP+(δejWSOP)loss+δejW NSOP+(δejWNSOP)loss}dρ,dv,dτ (29)
It
seems to me that this is the classical expression for the universe, which so
far has phenomenological content. To localize the equation it is necessary to
identify the mechanism of changes for each of its terms in both sides. For
resolving local problems of the universe we may turn to some equations, such as
equations for the mass flow, heat flow (entropy) and momentum transfer motion
flow. At the moment we have worked out the main goal – spontaneous and
non-spontaneous processes accounting losses for functions and functionals are
united in a single equation. Derivations for specific functions and functionals
are represented in our tutorial [8]. There is indirect statement that mass and
energy in above equations allowed as infinity (m=∞and U=∞). Similar
equations may be introduced to the global economy and biology.The equations for
mass flow, heat flow and entropy account the mechanism of various processes in
the system and the environment. For the flow system (such as the Gulf Stream,
for instance) these equations can be presented in different form as shown
further in this article.
The expression (10) does
not account mechanism of occurrence of thermodynamic processes.
Phenomenologically, mechanism of occurrence of thermodynamic processes in the
mass flow reflected in equations of the flow of energy in a form of mass flow,
energy flow and entropy, which includes convective flow, thermal conductivity,
heat transfer and energy accumulation within the flow due to internal
physicochemical processes.
The equations for mass
flow, energy in a form of heat and momentum for non-stationary and stationary conditions are presented further (accounting works of Dr. Usov). The
equation of the mass flowfor non-stationary conditions in
the3D-space, defined by x, y, z coordinates may be presented in the form of
five components: I – convection, II – diffusion, III – mass transition, IV –
physicochemical process and V – derivative, defining the flow state change with
respect to time.
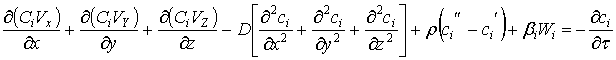 ,
,
I II III IV V
(30)
where: ci – concentration of matter
in the flow, x; y; z – coordinates, D – diffusion constant, β – mass transition constant, Vi – liner velocity of the
flow, τ – time, Wi – rate of passing of physicochemical
process
Under stationary conditions the derivative![]() and the equation (???) reduced to following
expression:
and the equation (???) reduced to following
expression:
div
(C I V)–DΔ2Ci+βΔCi+Wi=0 (31)
These
equations are accurate for the matter flow in a pipe with rigid walls and
without interaction with the environment.
Expressions
(30) and (31) are the mathematical basis for the development of kinetic models
of any physicochemical processes occurring in the flow, in stationary and non-stationary conditions, the
hydrodynamic modes of ideal displacement and ideal mixing and composite models,
as well as for fixed systems.
The equations of mass
flow supplemented with the kinetic equations, the dependencies of the diffusion
constants from T, the equations of mass transition, velocity constants with
respect to temperature and other terms.
The equation of
energy flow in the form of heat and entropy
Heat flow is induced to move by convection, heat
conduction, heat transfer, the occurrence of physicochemical process. Change of the state of flow occurring for
non-stationary conditions, which is represented in
the equation of the first derivative by heat over time.
The driving force of the flow is the temperature
difference over the area and its value. Higher the temperature difference
within the flow and between the flow and the environment, higher the value of
the first derivative and higher the change of the state of flow.
General equation, defining the change of the state of
heat flow with respect to change of temperature and time presented in the
following form:
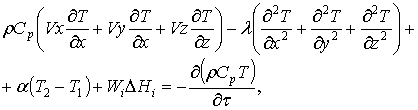 (32)
(32)
where: ρ – density of
matter flow, Cp – heat
capacity with p=const, x;y;z – flow
coordinates, T – temperature, λ – heat conduction constant, α – heat emission constant, Wi – rate of passing of physicochemical process, ΔHi – enthalpy of
physicochemical
process, τ – time, Vi – liner
velocity of the flow.
This expression is
accurate for non-stationary conditions of transition of energy flow in the form
of heat through the specified device. For stationary conditions the derivative
with respect to time vanishing.
![]() (33)
(33)
Thus the equation (???) is reduced to the
following form:
div(VρCpT)–![]()
![]() 2T+αΔT+WiΔÍ=0 (34)
2T+αΔT+WiΔÍ=0 (34)
or
div(VρCpT)–a![]() 2(ρCpT +b(ρCpÒ2–ρÑpT1)+Wi
ΔHi=0, (35)
2(ρCpT +b(ρCpÒ2–ρÑpT1)+Wi
ΔHi=0, (35)
where : a=![]() , b=
, b=![]() , assuming that the flow section is 1m2
, assuming that the flow section is 1m2
Introducing a substitution to the
expression (32):
ÑðT=Qp (36)
The resulting form is:
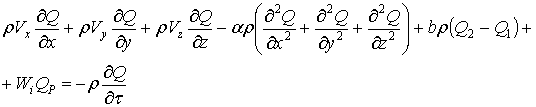 (37)
(37)
Dividing this by T will get us the following:
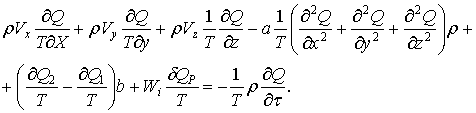 (38)
(38)
Including the concept of entropy, specifically
![]() , (39)
, (39)
derive the equation of the entropy flow in
the following form:
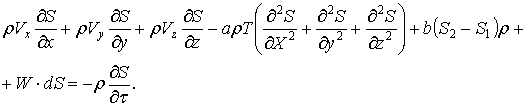 (40)
(40)
This is only accurate for entropy change within the matter flow.
Above equations is the basis
for thespeculative study of the technology of physicochemical
processes. Pairing corresponding terms of these equations may play important
role in the development of specific mathematical models for physical, chemical,
physicochemical and hydrodynamic processes.
Exposed matter flows,
energy in the form of heat flows and entropy flows can be described by the
equations, indispensably including the characteristics of internal and external
nature. It is essential to account the interaction of the flow (internal
system) and the environment (external system). These flows are studied in
thermodynamics of irreversible processes and thermodynamics of
spontaneous-nonspontaneous processes.
The momentum
equations
The momentum equation for
the flow (in particular theNavier–Stokes equations) defines the displacement of
particles within the flow of an incompressible fluid under the influence of
differences of velocities of shifting particles, gravity, pressure drop and
friction.In the projections of forces on the axes x, y, z motion of an
incompressible fluid can be presented for the non-stationary flow through the
following equations:
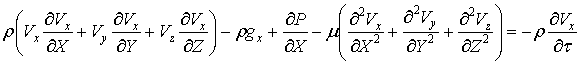 (???)
(???)
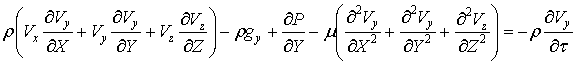 (???)
(???)
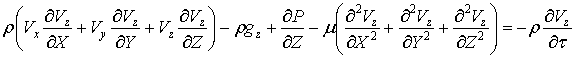 (41)
(41)
For stationary conditions the partial derivatives of the velocity of
the fluid particles with respect to corresponding time coordinateare vanishing:
![]() ,
, ![]() =0,
=0, ![]() =0. (42)
=0. (42)
Where: ρ – density, μ – mediumviscosity, V – velocity, g – gravity, P –
pressure, τ – time.
The equations presented in this chapterare the basis
for theoretical models of chemical and technological processes, processes for
oil and gas, transportation of oil and gas, the study of migration processes in
the lithosphere, water and air environments, as well as in biological systems.
These kinetic-thermodynamic equations can be used for local systems in the
universe, given the mechanism of the processes.
Sources
1.
Ð.Êëàóçèóñ.
Ìåõàíè÷åñêàÿ òåîðèÿ òåïëîòû.-  ñá. «Âòîðîå íà÷àëî
òåðìîäèíàìèêè»-Ì.:ÃÒÃÈ.-1934.
2.
Í.È.Áåëîêîíü. Òåðìîäèíàìèêà.-Ãîñýíåðãîèçäàò.-1954.-451ñ.
3.
È. Ïðèãîæèí. Ââåäåíèå â òåðìîäèíàìèêó íåîáðàòèìûõ
ïðîöåññîâ-Ì.:ÈÍË.-1960.-127 ñ.
4.
È.Ïðèãîæèí, Â.Êàíäåïóäè. Ñîâðåìåííàÿ òåðìîäèíàìèêà îò
òåïëîâûõ äâèãàòåëåé äî äèññèïàòèâíûõ
ñòðóêòóð.-Ì.:Ìèð.-2002.-461ñ.
5. Ð.Õààçå.
Òåîìîäèíàìèêà íåîáðàòèìûõ ïðîöåññîâ.- Ì.:Ìèð.-1967.-544 ñ.
6.
Ñ.äåÃðîîò, Ï.Ìàçóð. Íåðàâíîâåñíàÿ òåðìîäèíàìèêà.-Ì.:
Ìèð.-1964.-456 ñ.
7.
È.Ì.Êîëåñíèêîâ. Òåðìîäèíàìèêà ôèçèêî-õèìè÷åñêèõ
ïðîöåññîâ.- Ì.: ÃÀÍÃ èìåíè È.Ì.Ãóáêèíà.-1994.-238 ñ.
8.
È.Ì.Êîëåñíèêîâ. Òåðìîäèíàìèêà ôèçèêî-õèìè÷åñêèöåññîâ.-Ì.:
Íåôòü è ãàç.-2005.-480 ñ.
9. È.Ì.Êîëåñíèêîâ.
Òåðìîäèíàìèêà ðàâíîâåñíî-íåðàâíëîâåñíûõ ïðîöåññîâ.-Ì.: ÌÃÓ èìåíè
Ì.Â.Ëîìîíîñîâà.-2011.- 234 ñ.