Áèîëîãè÷åñêèå íàóêè/ 9. Áèîõèìèÿ è
áèîôèçèêà
Dzhimak S.S1,3., Basov A.A2., Artsybasheva O.M1., Barisheva E.V2., Bolotin S.N.,
Vlasov R.V.
1Kuban’ State
University, Russia
2Kuban’ State Medical University Minzdravsotsrazvitiya, Russia
3Laboratory of the Problems of Natural and New Materials, Southern
Scientific Center, Russian Academy of Sciences, Russia
ANTIRADICAL
ACTIVITY OF WATER WITH MODIFIED ISOTOPE COMPOSITION
Electron
paramagnetic resonance (EPR) is widely used to solve a number of
physicobiological problems [1-3]. It is also the main method for studying
paramagnetic particles in biological systems. Free radicals are paramagnetic
particles of biological importance. They help to regulate many intracell
processes [4], including immune mechanisms, the neutralization of xenobiotics,
apoptosis, and the metabolism of biologically active compounds. One promising
foodstuff for adjusting the antioxidant potential of an organism is water with
modified isotope composition (WMIC), e.g., water with a reduced deuterium
content [5].
Substituting
ordinary water for heavy lowers the electrical conductivity of electrolyte
solutions due mainly to an increase in viscosity and thus a reduction in ion
mobility. Heavy water mainly affects the active properties of an excitable
membrane. The presence of deuterium in biological systems leads to changes in
the structure and properties of DNA and proteins. At a 30% substitution of
ordinary water for heavy, the life processes of microorganisms stop and mammals
(e.g., laboratory rats) die [6].
In
the plasma of human and animal blood, the deuterium content slightly exceeds
its content in drinking water and is 140–160 ppm, depending on the habitat.
Water with modified isotope composition and a lowered deuterium content (WMIC
LDC) supposedly allows us to perform preventive maintenance and correct
oxidative stress, and thus to control the formation of free radicals in an
organism [7]. The aim of this study was to study the effect of the quantitative
deuterium content in the blood plasma and organs of laboratory animals on the
intensity of freeradical oxidation by NMR, EPR, and mass spectrometry under the
physiological conditions and in inflammatory processes.
One
of the most convenient methods for measuring the deuterium composition of blood
plasma is NMR spectroscopy [8]. However, this method does not allow us to
measure the deuterium content in the tissues of laboratory animal organs. This
problem was solved using an isotope mass spectrometer. EPR spectra were
registered in the X band at room temperature on a JES FA spectrometer 300
(JEOL, Japan). Water with reduced deuterium content was obtained on a setup
designed at Kuban’ State University [9]. The initial deuterium concentration in
the water was 40 ppm. The deuterium concentration in biological liquids was
determined on a JNM_ECA 400MHz pulse NMR spectrometer (JEOL). The isotope
composition of lyophilized organs of laboratory animals was determined on a
DELTA plus mass spectrometer (Finnigan, Germany).
 Three groups of rats (20 in each group) were used in our experiment. The
first was the control group, in which rats drank distilled mineralized water.
In the second, the rats drank distilled mineralized water with a deuterium
content of 40 ppm. In the third, the rats drank distilled mineralized water
with a deuterium content of 100 ppm. Once a week for three weeks, two-rats from
each group were euthanized to determine the deuterium content in the blood
plasma. Three weeks from the beginning of the experiment, oxidative stress was
stimulated by simulating a festering wound in the rats, using a two stage model
of oxidative stress [10]. Four weeks from the beginning of the experiment, the
rest rats were euthanized; their organs were lyophilized in an LS_1000
lyophilic dryer, and the paramagnetic center and deuterium contents were
determined on an EPR spectrometer and a mass spectrometer, respectively.
Three groups of rats (20 in each group) were used in our experiment. The
first was the control group, in which rats drank distilled mineralized water.
In the second, the rats drank distilled mineralized water with a deuterium
content of 40 ppm. In the third, the rats drank distilled mineralized water
with a deuterium content of 100 ppm. Once a week for three weeks, two-rats from
each group were euthanized to determine the deuterium content in the blood
plasma. Three weeks from the beginning of the experiment, oxidative stress was
stimulated by simulating a festering wound in the rats, using a two stage model
of oxidative stress [10]. Four weeks from the beginning of the experiment, the
rest rats were euthanized; their organs were lyophilized in an LS_1000
lyophilic dryer, and the paramagnetic center and deuterium contents were
determined on an EPR spectrometer and a mass spectrometer, respectively.
RESULTS AND DISCUSSION
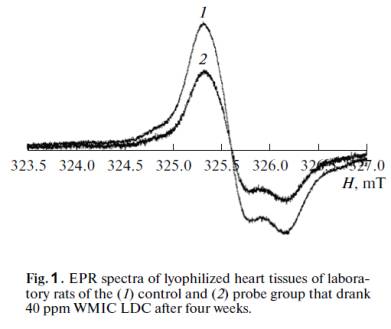
EPR spectra from lyophilized heart samples of
laboratory animals are presented in Fig. 1. They contain an anisotropic singlet
signal, the spin Hamiltonian parameters of which (g⊥ =
2.0074, g⎟⎪ = 2.003)
correspond to stable radicals [11–13].
 The EPR spectra of the liver and kidney samples were of a similar
nature. A pronounced antioxidative effect in the rats that drank water with a
residual deuterium content of 40 ppm was observed as early as the first week.
In lyophilized organs (liver, kidneys, heart), the number of paramagnetic
centers (according to the EPR data) fell by approximately 32–38%, relative to
the control group. This indicates a slowdown in the reduction of the free
radical numbers and confirms the favorable effect of light water on the
organism of animals. At the same time, a less pronounced antioxidative effect
was observed in rats that drank water with a residual deuterium content of 100
ppm: in lyophilized organs (liver, kidneys, heart), the number of paramagnetic
centers (according to the EPR data) fell by approximately 24–27%, relative to
the control group.
The EPR spectra of the liver and kidney samples were of a similar
nature. A pronounced antioxidative effect in the rats that drank water with a
residual deuterium content of 40 ppm was observed as early as the first week.
In lyophilized organs (liver, kidneys, heart), the number of paramagnetic
centers (according to the EPR data) fell by approximately 32–38%, relative to
the control group. This indicates a slowdown in the reduction of the free
radical numbers and confirms the favorable effect of light water on the
organism of animals. At the same time, a less pronounced antioxidative effect
was observed in rats that drank water with a residual deuterium content of 100
ppm: in lyophilized organs (liver, kidneys, heart), the number of paramagnetic
centers (according to the EPR data) fell by approximately 24–27%, relative to
the control group.
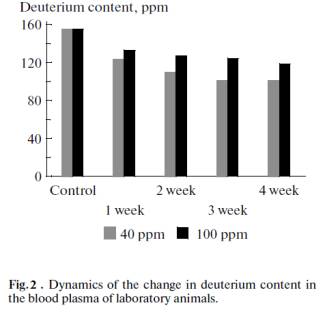
The dynamics of the change in the deuterium content
during the experiment in the blood plasma of laboratory animals consuming water
with residual deuterium contents of 40 and 100 ppm is shown in Fig. 2. It can
be seen from in the figure that the deuterium content in the blood plasma of
laboratory animals according to the NMR spectroscopy data gradually declines
and reaches a plateau after three weeks of using WMIC LDC.
 The deuterium content in the lyophilized tissues of liver, kidneys, and
heart of laboratory animals that drank WMIC LDC for a month is given in Fig. 3.
It can be seen from Figs. 2 and 3 are that the deuterium concentration fell to
a lower level in plasma and tissues when water with a lower deuterium
concentration was consumed. When water with deuterium concentrations of 100 ppm
and 40 ppm was consumed, however, the plateau of deuterium concentrations in
plasma and organs was reached in three weeks after the first use of WMIC LDC.
According to the EPR spectroscopy data, water with a residual deuterium content
of 40 ppm reveals faster development of the antioxidative effect during the
development of festering inflammatory diseases in laboratory animals. This is
related to a sharp increase in the immunity and resistivity of the organism.
The deuterium content in the lyophilized tissues of liver, kidneys, and
heart of laboratory animals that drank WMIC LDC for a month is given in Fig. 3.
It can be seen from Figs. 2 and 3 are that the deuterium concentration fell to
a lower level in plasma and tissues when water with a lower deuterium
concentration was consumed. When water with deuterium concentrations of 100 ppm
and 40 ppm was consumed, however, the plateau of deuterium concentrations in
plasma and organs was reached in three weeks after the first use of WMIC LDC.
According to the EPR spectroscopy data, water with a residual deuterium content
of 40 ppm reveals faster development of the antioxidative effect during the
development of festering inflammatory diseases in laboratory animals. This is
related to a sharp increase in the immunity and resistivity of the organism.
CONCLUSIONS
The change in the deuterium content in the plasma and
lyophilized tissues of organs of laboratory animals was analyzed on the basis
of present day spectroscopy methods. EPR spectroscopy was used to find that,
depending on the deuterium concentration in the consumed water, the number of
paramagnetic centers in investigated lyophilized tissues of heart, liver, and
kidneys in the case of WMIC LDC fell by 24–38%, relative to the control group.
This testifies to the considerable effect of small fluctuations in the
concentration of deuterium in the surrounding medium on the ability of an
organism to adapt.
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for
Basic Research, project no. 11_04_96523_r_yug_ts; and by the RF Ministry of
Education and Science Grant nos. 4.1755.2011, 7.369.2011.
REFERENCES
1.
Ingram, D.J.E., Biological and
Biochemical Applications of Electron Spin Resonance, London: Adam Hilger
Ltd., 1969; Moscow: Mir, 1972.
2.
Azhipa, Ya.I., Mediko_biologicheskie
aspekty primeneniya metoda elektronnogo paramagnitnogo rezonansa (Biomedical
Aspects of Electronic Paramagnetic Resonance Method), Moscow: Nauka, 1983.
3.
Saprin, A.N., et al., in Aktual’nye
voprosy sovremennoi onkologii (Topical Problems of Modern Oncology),
Sukhareva_Nemakova, N.N., Ed., Moscow: Izd. MGU, 1970.
4.
Vladimirov, Yu.A., Biokhimiya,
2004, vol. 69, no. 1, p. 53.
5.
Olariu, L., et al., Lucr ri Stiin
ifice Medicin Veterinar , 2007, vol. 40, p. 265.
6.
Lobyshev, V.N. and Kalinichenko,
L.P., Izotopnye effekty D2O v biologicheskikh sistemakh (D2O Isotopic
Effects in Biological Systems), Moscow: Nauka, 1978.
7.
Olariu, L., et al., Lucr ri Stiin
ifice Medicin Veterinar , 2010, vol. 43, no. 2.
8.
Baryshev, M.G., et al., Ekologich.
Vestn. Nauchn. Tsentrov ChES, 2011, no. 3.
9.
Baryshev, M.G., et al., Nauka
Kubani, 2010, no. 3, p. 18.
10.
10. Basov, A.A., RF Patent
Application 2011100352/14 (000483) IPC G01N33/48, 2012
11.
Sovremennye metody biofizicheskikh
issledovanii (Modern Methods in Biophysical
Researches), Rubin, A.B., Ed., Moscow: Vysshaya shkola, 1988.
12.
Borovik, E.S., et al., Lektsii po
magnetizmu (Lectures on Magnetism), Moscow: Fizmatlit, 2005.
13.
Pulatova, M.K., et al., Elektronnyi
paramagnitnyi rezonans v molekulyarnoi radiobiologii (Electron Paramagnetic
Resonance in Molecular)