Біологічні науки/10. Генетика і цитологія
або
/11. Біоінженерія і біоінформатика
Inna Bogaichuk
Department of Biomedical Engineering, NTUU ‘KPI’
HEALTH AND SOCIETAL BENEFITS
PRESENTED BY STEM CELL RESEARCH
Stem cell research is a field that has generated much
activity in laboratories, media offices and higher courts. Parallel to the
potential new treatments for incurable diseases and opportunities for
bioentrepreneurs, heated ethical and legal debates have arisen around the
world. This compilation of information illustrates valuable track record in the
area of stem cell research and highlights the need to continue to fund this
research so that its full potential can be realised. Stem cell research holds a
strong potential to deliver new treatments for serious diseases and injuries
for which today few effective treatments exist. Great hope is invested in this
field by researchers, based on the expectation that we will learn how to
replace damaged cells in patients with new, healthy cells grown or produced in
the lab, or by inducing organ regeneration from stem cells in the body. The
field has attracted priority status in many countries and has advanced rapidly.
Indeed, some basic research findings are now being translated into new
treatments. Furthermore, new drugs are identified and tested, and potentially,
the way that cells can be generated in the lab. Stem cells, whether they occur
in the body or in the lab, are defined by two cardinal properties: they can
self-renew (generate perfect copies of themselves upon division) and
differentiate (produce specialized cell types that perform specific functions
in the body).
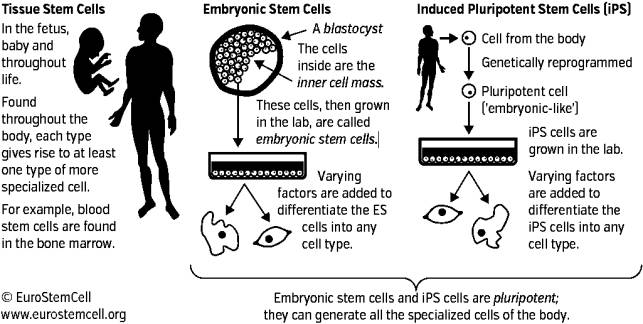 Figure 1. Stem cells and their types
Figure 1. Stem cells and their types
The promise of stem cells as new tools for benefiting
human health resides in these twin properties that, in principle, allow
production of unlimited quantities of defined cell types (e.g., for use in drug
screening or transplantation). Stem cell research has the potential to improve
and accelerate drug screening, drug discovery, and pre-clinical toxicological
assessment of new drugs. Controlled differentiation of human pluripotent cells
and/or ex vivo expansion of human tissue stem cells could produce unlimited
supplies of defined human cell types. Once developed, this technology should
permit screening of more compounds in shorter time and at less expense than is
currently possible.In addition to cell replacement strategies, increased
understanding of the intrinsic regenerative potential of individual organs,
coupled with knowledge of how to control the scarring response in damaged
tissues, may allow the development of drugs aimed at stimulating the body’s own
(endogenous) stem cells to initiate or enhance repair. This approach is
expected to prove more suitable than cell replacement for some diseases.
Finally, where use of a patient’s own cells is not
possible, either because a sufficient number of cells cannot be obtained or
because protocols for generating the required cell type in the lab (e.g., from
iPS cells) have not yet been developed, the consequences of immunosuppression
must also be considered. Banking hES and iPS cell lines is one way to ensure
that patients can receive cells with a good immunological match, thus
minimizing any required immunosuppression. While cell replacement offers hope
for the treatment of many diseases in the long term, it may still be some time
before large-scale clinical use is available for most applications.
Understanding how to produce many of the specialized cell types in vitro
remains a major hurdle. Furthermore, the field faces challenges around quality
control. It is essential that only defined cell populations are introduced into
patients; this requires careful characterization of the cell populations
intended for transplantation, in terms of gene expression and epigenetic
profiles and functional attributes, and also to ensure that the populations do
not contain other potentially harmful cell types. For cells generated from
human pluripotent cells, for example, contamination of the transplant
population with even a small number of residual ES or iPS cells could promote
tumor formation. In recent years, stem cell research has grown and changed
remarkably. Many nations around the world are contributing to stem cell
research; the dynamic nature of the field suggests the landscape is likely to
shift as new players develop research programs and refine their expertise. Some
clinical applications of tissue stem cells are already well established and
importantly, some experimental pluripotent cell treatments are in clinical
trials. However, if tissue and pluripotent stem cells are to fulfil their
promise of meeting unmet medical needs, the challenge to further foster a
regulatory, funding and corporate environment that facilitates the process of
taking laboratory developments towards the clinic will be of major importance.
References:
1. Stem Cell
Research: Trends and Perspectives on the Evolving International Landscape. – Режим доступу: http://www.eurostemcell.org
2. Human stem cells, cloning and
research.
– Режим
доступу: http://www.tenk.fi/sites/tenk.fi/files/HumanStemCellsCloningandResearch.pdf
3. Microengineered hydrogels for
stem cell bioengineering and tissue regeneration. – Режим доступу: http://www.harvardclub.org.au
4. Global assessment of stem cell
engineering . – Режим доступу: http://www.wtec.org/SCE/StemCellEngineering-Final-1.25.13.pdf