N.
S. Alekseeva, T. I. Los’, L. M. Antypenko
Zaporizhzhya
State Medical University
Organic
and Bioorganic Chemistry Department
Docking studies of the 2-([1,2,4]triazolî[1,5-c]quinazolin-2-ylsulfanyl)acetamides to Candida albicans dihydrofolate
reductase
Candida albicans is one of the leading causes among microbial infection in the hospital
setting, and opportunistic fungal infections present a major problem to AIDS
patients. One of the ways to develop new therapeutically useful agents against
fungal growth is synthesis of dihydrofolate reductase (DHFR) inhibitors. [1] Assuming
the fact, that 7-(pentan-3-yl)-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine is reported to
has affinity to DHFR and structurally resembles synthesized 2-([1,2,4]triazolî[1,5-c]-quinazolin-2-ylsulfanyl)acetamides; and also because broad spectrum antibiotic
trimethoprimt has a striking selectivity for DHFR, docking studies of above
mentioned triazoloquinazolines could predict the possibility of antimicrobial and
antifungal activity presence as well as it’s mechanism.
The structure of all synthesized compounds was confirmed by elemental
analysis and their spectral data (FT-IR, LC-MS and 1H-NMR spectra).
The molecular docking study was performed using geometry
optimized structure of the compounds into the active site of DHFR (1AI9 and 1AOE, for the
holoenzyme and the inhibited enzyme structure, respectively) [2]. Investigation was conducted by flexible molecular docking using the
software package OpenEye [3,4]. The obtained scoring functions (Shapegauss,
PLP, Chemgauss2, Chemgauss3, Chemscore, OEChemscore, Screenscore, CGO, CGT,
Zapbind, Consensus Score) indicate the best possibility of the matching into
the ligand-protein complex. The 7-(pentan-3-yl)-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine
(GW345) was used as the reference [2].
Molecular
docking with visual inspection indicated that 2-([1,2,4]triazolo[1,5-c]quinazolin-2-ylthio)-N-(5-(2-(3-chlorophenylamino)-2-oxoethylthio)-1,3,4-thia-diazol-2-yl)acetamide
was bound to the DHFR
with hydrogen bond: N···NH/ARG 79B (2.50 Å). According
to Consensus score it showed
the highest affinity to DHFR, but even better comparing with reference compound.
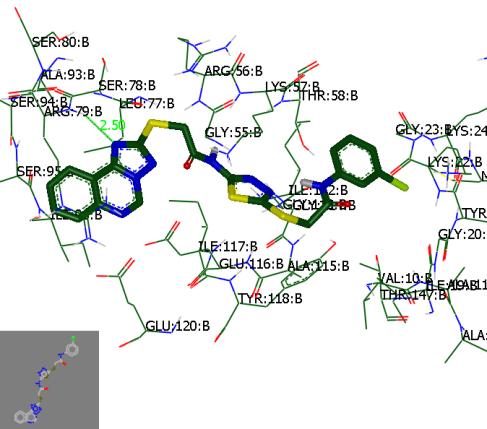
Figure. Interaction of the substance with the
binding site of DHFR; H-bond is shown as
dotted line
As a result, among investigated 2-([1,2,4]triazolî[1,5-c]quinazolin-2-ylsulfanyl)acetamides
there were found substances with high affinity to DHFR.
The antifungal activity of the ones with best Consensus Scores will be
investigated in vitro.
Literature:
1. X-ray crystallographic studies of Candida albicans dihydrofolate
reductase. High resolution structures of the holoenzyme and an inhibited
ternary complex. M Whitlow, A J Howard, D Stewart, K D Hardman, L F Kuyper, D P
Baccanari, M E Fling, R L Tansik. J. Biol. Chem. 12/1997; 272(48):30289-98.
2. Protein Data Bank, pdb. [http://www.pdb.org].
3. Virtual Screening in Drug Discovery, (Eds.: J. Alvarez, B. Shoichet),
CRC Press, Taylor & Francis, Boca Raton, FL, 2005, 451.
4. Fred Receptor2.2.5, Vida4.1.1, Flipper, Babel3, Omega2.4.3 and Fred2.2.5:
OpenEye Sci. Soft. Inc. Santa Fe, NM, USA; 2011. [http://www.eyesopen.com].