INVESTIGATE
MUTATION GENE OF BETA CHAIN FIBRINOGEN (FGB) IN A THROMBOPHILIA PATIENT WITH
ACUTE MYOCARDIAL INFARCTION
Trang Thi Nguyen,1
Phan Duc Tran1, Huong Thanh Truong 1, Anh Thi Lan
Luong1,
Binh Thanh Le2, LyThi Minh Nguyen1, An Hong Le3
1- Hanoi Medical University
2- Vietnam Heart Institute, Bachmai Hospital
3-Chu Van An high school
Hanoi
Medical University, Department Biomedicine and genetics, 1, Ton That Tung, Dong
Da, Ha Noi, Viet Nam. Tel: 04.38523798;
Fax:04.38525115. trangtrang1182@yahoo.com.
ABSTRACT
Objectives: To investigate mutation
on promoter region of fibrinogen beta chain gene in thrombophilia patient with
acute myocardial infarction (AMI). Subjects: A 50 year - old male
patient, history of left leg deep vein thrombosis; was diagnosed of AMI.
Coronary angiogram: thrombosis caused total occlusion of the middle region of
right coronary artery (RCA), middle region of
left circumflex coronary artery (LCx), middle segment of left coronary artery
(LAD) and partial thrombotic occlusive the distal end of the left main coronary
artery and the proximal segment of LAD. Methods:
clinical examination, lab tests, echocardiography, emergency angiogram,
sequencing beta chain fibrinogen binding gene, using
reference and control. Results:
find out point mutation C148T at promoter region of FGB gene. Conclusion: following a case of AMI
thrombophilia patient with C148T mutation on FGB gene, we aim to develop a
research in a wider population of familial myocardial infarction patients in
order to find out a relationship between genetic polymorphism of the fibrinogen
gene, the corresponding plasma level of fibrinogen and the risk for clinical
event (AMI).
Key
words:
fibrinogen, acute myocardial infarction (AMI), FGB gene, promoter.
INTRODUCTION
Myocardial infarction
(MI), one of the leading causes of death worldwide, is a complex disorder
influenced by multiple genetic and environmental factors. Thrombosis is
generally accepted as the most common pathogenetic pathway of acute MI. As the
last target of coagulation, fibrinogen plays a crucial role in the
process of thrombus
formation and evolution, and elevated plasma fibrinogen levels are strongly
associated with the risk for MI1. Fibrinogen is a glycoprotein consisting of two subunits of three
distinct polypeptide chains (Aα, Bβ, and γ). Each of the chains
is encoded by a different gene situated on the long arm of chromosome 4 at 4q23-32. It has been
demonstrated that the synthesis of the Bβ chain is the rate limiting
factor in the production of the mature fibrinogen protein3. Thus, most studies focus on the association of
polymorphisms in the fibrinogen β-chain (FGB) gene with MI. Several polymorphisms
in the FGB gene were found to be associated with increased plasma fibrinogen
levels4-6. But the role of the FGB polymorphisms as a risk factor of
MI has been debated7.
In view of the conflicting
findings, we performed an association study to assess the effect of common
genetic variants in FGB gene on the risk of MI in a Viet Nam population. We
hereby report a case of acute myocardial infarction; the location was easy to
form blood clots that can detect changes in the promoter region of the FGB gene.
METHODS
Subjects
A 50 year old male patient
admitted to hospital because of chest pain at the 10th hour of onset.
The patient has a history of heavy smoking (1 packet per day for 30 years). He
has also suffered from deep vein thrombosis of the left leg since Sept 2010 and
is on treatment with Sintrom (Acenocoumarol) with 1mg per day.
On admission, the patient was
examined, conducted functional diagnostic testings, finally came with a
definitive diagnosis of acute myocardial infarction (MI). He also was taken
blood for genetic tests. The patient’s blood sample was stored in a test tube with EDTA as an anticoagulant and obtained for
genetic studies of FGB gene at the Department of Biology and Medical Genetics,
Hanoi Medical University, Vietnam. A control blood sample for comparison was
taken from a healthy man of that age with the patient with no history of
myocardial infarction or angina pectoris.
Diagnostic Imagings and Pre-clinical tests
Patient was checked by doppler echocardiography, emergency coronary angiography and 64 - slide computed tomography of the chest.
Pre-clinical tests were done in his bood, including coagulation basic, protein
C, protein S, antithrombin III, lipid profile, blood glucose, liver function,
kidney, etc.
Genotyping
Genomic DNA was extracted from peripheral blood by
phenol - chloroform method and stored at -20°C until analysis. The DNA was purified and checked by the
amount of spectrophotometer nano-drop. FGB gene was replicated by standard
polymerase chain reaction (PCR) in the promoter region of a specific nucleotide
position which is determined by international bank sequences (GenBank, M
64983,1). Specific primers used for the promoter region of the FGB, forward:
5’-AATAACTTCCCATCATTTTGTCCAATTCC-3’, reverse:
5’-AGTCGTTGACACCTTGGGACTTAACTTG-3’ (primer pairs FGBf3m- FGBr4m). For PCR amplification, the standard program was used as follows: an initial
denaturation step at 95°C for 5 min was followed by 40denaturation cycles of 30
s at 95°C, 30 s of annealing at 55°C, and 30 s of extension at 72°C, and at
last followed by a final elongation cycle at 72°C for 5 min. PCR reactions were
carried out in a final volume of 50 µl containing genomic DNA template,
primers and the reaction components
for PCR (Taq DNA polymerase, dNTPs, MgCl2, dH2O). PCR products were purified by
PureLinkTM kit (Invitrogen).
Sequencing
FGB gene was performed on a Genetic Analyzer ABI PRISM 3100. The quality
parameters and the peak were collected, tested by the ABI Data Collection
software v2.0 and Sequencing Analysis v5.3 Software. The sequence of the
promoter region of the FGB gene case and control in Vietnam were compared with
reference sequences, which is published in GenBank by using analysis software
Chromas Lite v2.1.1 and Seaview to identify point mutations.
RESULTS
Clinical characteristics
of patients with myocardial infarction
On admission,
the patient still had chest pain, heart rate 120 bpm and blood pressure 90/60
mmHg (both arms), fine crackles at the base of both lungs, mild, tender
hepatomegaly (congestion).
Electrocardiography
(ECG): sinus tachycardia, ST segment
elevation in leads DII, DIII, aVF, and from V3 to V6.
Emergency
Echocardiography: LVDd 52mm,
LVDs 41mm, EF Simpson (4 chamber view) 53%, (2 chamber view) 14%, hypokinetic
movement of different left ventricle
(LV) wall regions, with more profoundly in the inferio - posterior region of
the LV wall. There was mild pericardial effusion.
The patient
was treated with drugs for acute MI. Emergency coronary angiography was
indicated for him.
Pre-clinical
characteristics of patients
Analyses determined the state of the heart muscle
necrosis due to myocardial infarction:
troponin T, CK and CK MB increased up to 8 ng / mL, 9600 IU / mL, 1100 UI / L,
respectively. ProBNP increased with time up to nearly 1700 pmol / l (expressed
condition with severe heart failure), GOT and GPT rose to 823 and 127 IU / l.
The
coagulation tests of the patient were closely monitored over time to see the
changes in blood clotting, namely: PT% reduction in speed slightly less than
20% (17.2%; INR increased to 3.35 (patient receives anticoagulant vitamin K antagonists
for the treatment of the left leg deep vein thrombosis); APTT was abnormal with
a value ranging from 174ms - 79.5 ms - 42 ms - 28ms makes APTT disease outcome
/ evidence also ranged from 5.5 to 0.99 (the highest stage additional factors
often using continuous infusion of heparin after fibrinolytic therapy. Protein C
47,4% (↓), protein S 22,7% (↓), Antithrombin III: 96,6%
(normal) (Blood samples were taken to test the condition of patients still
using anticoagulant vitamin K antagonist). Fibrinogen 4.7 g / l (up slightly).
Other preclinical studies: platelet count was reduced to 103.000 cell /ml;
Bilan lipids, blood glucose, renal function were within normal ranges.
Emergency coronary
angiography images: complete
occlusion of the 2nd segment of the right coronary artery, the circumflex coronary artery and the left anterior descending
coronary artery. Angiography revealed a thrombotic sub-total occlusion
of left main coronary artery and the 1st
segment of the left descending coronary artery.
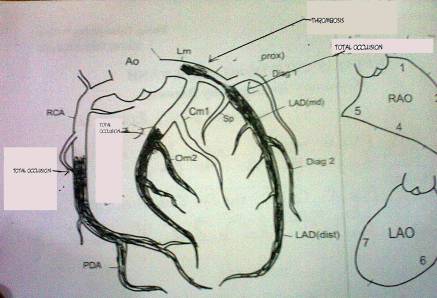
Figure
1. Coronary angiography image of the patient
With
the above results of the coronary angiography, team of interventionists decided
to stop the procedure. The patient was transfered back to the cardiac care unit
(CCU), prepared for receiving thrombolytic therapy. Alteplase was the drug of
choice. The protocol was as followed: bolus 15mg, then 0.75 mg/kg within 30
minutes, and then 0.5 mg over 60 minutes. Continuous intravenous heparin
infusion was given at the same time at the rate of 1000UI/h, and also
dobutamine infusion at the dose of 5 mcg/kg/min. the patient’s hemodynamics was
stable during fibrinolytic therapy.
After
fibrinolysis, the patient’s chest pain was improved. The following days, the
patient was being medically treated intensively for thrombotic disease,
coronary artery disease and heart failure. The anticoagulant and anti-platelet
aggregation drugs used include: aspegic 200mg/day, clopidogrel 75 mg/day,
anticoagulant- antagonist vitamin K: sintrom 1mg/day.
The patient was reevaluated with Doppler echocardiography after 3 days in hospital: LVDd 55mm, LVDs 44mm, EF simpson (2B) 32%, moderate pulmonary artery
hypertension; akinesis of the LV wall region, supplied by left anterior
descending and right coronary artery; mild pericardial effusion. Doppler
echocardiography of the lower limbs revealed old thrombosis of the left
iliofemoral vein which was partially revascularized.
In the 64 slide MSCT revealed no pulmonary artery
infarction, there was pleural effusion both sides mildly.
The
patient was discharged after 2 weeks in better physical condition: heart failure
improved, no sign of chest pain. Patients continued medical therapy 2 weeks,
improved heart failure, chest pain disappeared.
The patient readmitted after 2 weeks: full stability, no chest pain, myocardial tests for cell injury was in
normal ranges.
Mutation C148T of FGB gene in a
patient with MI
PCR
amplifying FGB promoter
regions
The extracted genomic DNA was used for the matrix in
the replication FGB promoter. To analyze the changes in the promoter region of
the FGB up to 728 bp, we used primers FGBf3m-FGBr4m. The amplified products were detected on
1.5% agarose gel, along with a 100-bp DNA ladder. The result of electrophoresis
of product PCR (Figure 2) showed the specificity and quality of the isolated
DNA.
1 - Control
sample 2 -
Case sample 3
- Ladder
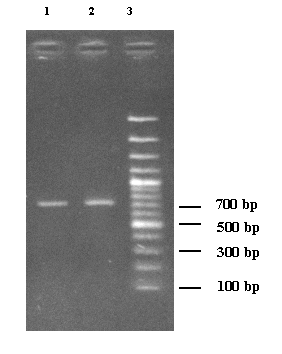
Figure 2. Electrophoresis of products PCR patients and
control samples.
Ladder: molecular weight marker 100 bp
Promoter
sequences
PCR products were sequenced after purification in both directions
(Forward - F, reverse - R), compared to the control sequences in the GenBank,
we found at nucleotide position -148 promoter region of a patient with FGB gene
point mutations such as nucleotide Cytozine (C) is replaced by thymine (T),
designated -148C à T or C148T (Fig. 3).
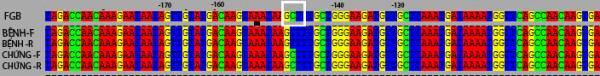
Figure 3. Comparison of nucleotide sequence of the
promoter region of the FGB in control and case samples
On the forward and reverse chain in FGB in case (Patient-F and
Patient-R) found a point mutation that C àT, TT genotype. Meanwhile, CC genotype was confirmed in the control.
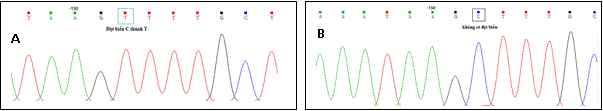
Figure 4. Peaks of nucleotides in the promoter region
of the FGB
A: Case - sample with
mutation -140CàT. B:
mutation is not found
in
Control
DISCUSSION
Patients aged 50 years with
a history of smoking and thrombosis of deep veins of the left leg, continuing
anticoagulant therapy – a vitamin K antagonist (INR = 2.1). He was hospitalized
with acute MI. Coronary angiography revealed three trunks thrombosis coronary
artery. Clinical assays showed a decrease in the platelet count, coagulation
outer (PT%, INR) in the limit of a vitamin K antagonist therapy, fibrinogen
levels in normal (2-4 g/l), a decrease ratio of protein C, S (but the patient
still taking vitamin K antagonists when had blood withdrawal for analysis). In
situations where the patient has arterial and venous thrombosis, wherein the
clinical coagulation assays showed changes are unknown, we doubt that may be
part of the development of the disease the patient is affected by other factors
including genetic factors? On the basis of the scientific literature in the
world, we decided to take a patient's blood for genetic diagnostics, focusing
on genes encoding fibrinogen, especially because the FGB has quite a lot of
research on the world's stock is the relationship between polymorphisms of these
genes and cardiovascular risk factors in general, the risk of coronary heart
disease in particular.
As a result of the genetic studies of polymorphic markers promoter
region of the FGB in patients compared with sequences control samples was
detected C148T mutation in the promoter region of the FGB. At the Center of
stroke prevention in Australia, a study demonstrates the first evidence of a
significant association between the T/T148 genotype at the b-fibrinogen gene
and carotid atherosclerosis8.
Ewa Wypasek et al (2012) conducted analysis FGB 243 patients with
myocardial infarction, coronary bypass surgery (coronary artery bypass grafting
- CABG), found 101 patients with mutation C148T, that has genotype CT or TT.
They found that CT+TT genotype is an independent predictor of high
postoperative CRP levels in CABG patients. Carrying of the -148T allele has
also been associated with increased postoperative IL-6 levels in CABG patients9.
Looking at the results of our investigation in the case with mutation C148T,
the patient has the TT genotype. This patient is treated with sintrom daily,
although fibrinogen levels on admission in the normal range, then increased.
However, a history of venous thrombosis and were acute myocardial infarction
with thrombosis of three trunks of the coronary arteries. This may indicate
that the C148T mutation may lead to a breach of the synthesis reaction of
fibrinogen that influence inflammation and blood clotting.
Detection of C148T mutation in a
patient with acute MI with being easy formation of blood clots suggests that we
need to expand the study of association of polymorphisms of FGB with MI in
Vietnam to determine the relationship between genotype and phenotype or
pathological manifestations. In fact, until now, modification of the gene
considered polymorphism FGB and scientists explore these polymorphisms with
respect to the pathological manifestations. Behague I. et al evaluated the
association between genetic polymorphisms in the FGB gene and the severity of
coronary artery disease (CAD) in patients with familial acute MI show that among
11 variants of the β fibrinogen gene that were
investigated in the ECTIM study, 8 were mutually very tightly or completely
associated to the concentration of fibrinogen in the plasma, especially in smokers and patients with
MI7.
Some studies have shown strong correlations between polymorphism
genotype of the gene for the beta chain synthesis and plasma fibrinogen
concentrations.
For example, polymorphism of the gene beta fibrinogen was detected at 3 ' position by restriction enzyme
BclI (polymorphism BclI) as polymorphism allele pairs with low frequency of B2,
only 0.18 in the general population. B2B2 genotype is rare, but was associated
with increase levels of fibrinogen in blood serum 15% - 20% compared with the
genotype B1B111.
In addition, studies of Van’t Hooft F., Sekar K. and his colleagues showed
polymorphisms G455A and G854A gene beta fibrinogen significantly influenced by
the concentration of serum fibrinogen. G455A mutation of a gene beta fibrinogen is most associate with increase levels of fibrinogen, found
in both sexes in society as a whole. This study has also shown a link between
the C148T and G455A mutations,
in pathological cases, usually appears one of the two mutations7,11-12.
Although the relationship polymorphisms FGB with the risk of cardiovascular
diseases in general and coronary heart disease risk in particular still has
differences but research FGB gene has continue with time and detecting each
patient explaining on
pathological mutations or polymorphisms.
In conclusion, new areas of
research that we would like to mention the case of the C148T mutation FGB gene
in a patient with MI being easy for the formation of pathological thrombosis opens new research
direction in the most general population of patients with myocardial
infarction, family, nature, understanding the relationship between genotype
polymorphism on regulation synthesis fibrinogen, the response of serum
fibrinogen levels, as well as a risk of clinical events (acute myocardial
infarction). These links will serve as a basis for studying the etiologic role
of fibrinogen in acute MI,
as well as the basis for evaluation of interventions that can be made for
incident heart attack in patients with familial mutation arising properties and genetic counseling for carriers.
REFERENCES
1.
Wilhelmsen L. et al. Fibrinogen as a risk
factor for stroke and myocardial infarction, The New England Journal of Medicine, 1987;11: 501-505.
2.
Kant, J.A., A.J. Fornace Jr., D.
Saxe, M.I. Simon, O.W. McBride, and G.R. Crabtree. Evolution and organization
of the fibrinogen locus on chromosome 4:Gene duplication accompanied by
transposition and inversion. Proceedings of the National Academy of Sciences of
the United States of America, 1985; 82: 2344–2348.
3.
Yu S, Sher B, Kudryk B, Redman CM.
Fibrinogen precursors: order of assembly of fibrinogen chains. J Biol Chem,
1984; 259: 10574-10581.
4.
Gardemann A, Schwartz O, Haberbosch
W, Katz N, Weiss T, Tillmanns H, et al. Positive
association of the beta fibrinogen H1/H2 gene variation to basal fibrinogen
levels and to the increase in fibrinogen concentration during acute phase
reaction but not to coronary artery disease and myocardial infarction. Thromb
Haemost, 1997; 77: 1120-1126.
5.
Kathiresan S, Yang Q, Larson MG,
Camargo AL, Tofler GH, Hirschhorn JN, et al. Common genetic variation in five
thrombosis genes and relations to plasma hemostatic protein level and
cardiovascular disease risk. Arterioscler Thromb Vasc Biol, 2006; 26:
1405-1412.
6.
Reiner AP, Carty CL, Carlson CS,
Wan JY, Rieder MJ, Smith JD, et al. Association between patterns of nucleotide
variation across the three fibrinogen genes and plasma fibrinogen
levels: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J
Thromb Haemost, 2006; 4: 1279-1287.
7.
Behague I, Poirier O, Nicaud V,
Evans A, Arveiler D, Luc G, et al. Fibrinogen gene polymorphisms are associated
with plasma fibrinogen and coronary artery disease in patients with myocardial
infarction: The ECTIM Study. Circulation, 1996; 93: 440-449.
8.
Schmidt H. et al. Beta-fibrinogen
gene polymorphism (C148T) is associated with carotid atherosclerosis:results of
the Austrian Stroke Prevention Study. Arterioscler Thromb Vasc Biol, 1998;18:
487-492.
9.
Ewa Wypasek et al. Fibrinogen Beta-Chain -C148T
Polymorphism is Associated with Increased Fibrinogen, C-Reactive Protein, and
Interleukin-6 in Patients Undergoing Coronary Artery Bypass Grafting. Inflammation, 2012; 35(2): 429-435.
10.
Francesco Z. et al. BclI
polymorphism in the fibrinogen beta chain gene is associated with the risk of
familial myocardial infarction by increasing plasma fibrinogen levels. A case
control study in a sample of GISSI-2 patients. Arteriosclerosis, thrombosis and
vascular biology, 1997; 17: 3489-3494.
11.
Sekar K. et al. Common Genetic Variation in Five Thrombosis
Genesand Relations to Plasma Hemostatic Protein Leveland Cardiovascular Disease
Risk. Arterioscler Thromb Vasc Biol., 20062; 6:1405-1412.
12.
Van’t Hooft F. et al. Two common, functional polymorphisms in the
promoter region of the beta-fibrinogen gene contribute to regulation of plasma
fibrinogen concentration. Arterioscler Thromb Vasc Biol.; 1999; 19: 3063-3070.