Zh.E.Ibrayeva1,2,
S.E.Kudaibergenov1,2
1) Kazakh National
Technical University n/a K.I. Satpaev, Kazakhstan
2)Institute of Polymer
Materials and Technology, Kazakhstan
SEPARATION OF PROTEIN MIXTURES BY
POLYAMPHOLYTES
INTRODUCTION
Studying of polyampholyte-protein complexes (PPC) is
important from the biochemical and biotechnological point of view. Complexation
of polyampholytes and proteins can be used for separation and purification of
latter, immobilization and stabilization of enzymes. [1]. Commonly used methods
for separation of proteins are chromatography, precipitation of proteins by
inorganic salts, two-phase precipitation of proteins that is based on uniform
distribution of proteins between two aqueous polymer phases [2,3]. The present
communication considers the behavior of PPC formed between the water-soluble
“annealed” polyampholytes and bovine serum albumin and lysozyme.
EXPERIMENTAL
Copolymers of
dimethylaminoethyl methacrylate and methacrylic acid (DMAEM-MAA) were
synthesized by radical copolymerization.
The compositions of polyampholytes (PA) determined by back
potentiometric and conductimetric titrations are equal to [DMAEM]:[MAA]=38:62
mol% (PA-I) and [DMAEM]:[MAA]=62:38 mol % (PA-II). Intrinsic viscosities of PA-I and PA-II
are equal to [h]=3,7 dL/g in 0,01
N HCl and [h]=0,68 in 0,1 M KCl,
respectively. The isoelectric points
(IEP) of polyampholytes found from the viscometric data are equal to 5.0 for PA-I
and 8.6 for PA-II. Bovine serum albumin (BSA) and Lysozyme purchased
from the Sigma Chemical Co. had isoionic points (IIP) at pHIIP=4,7
and pHIIP=11,0. The intrinsic viscosities of BSA and Lysozyme are
equal to 0,08 and 0,07 dL/g, respectively. Reagent-grade KCl, NaOH, HCl and
solvents were used. Potentiometric and conductimetric titrations were carried
out on the pH/conductivity meter “Mettler Toledo MPC 227” (Switzerland). The
viscosity of the solutions was measured in an Ubbelohde viscometer. The
composition of PPC was expressed as mass ratio of protein to polyampholytes (Mprot:MPA).
RESULTS AND DISCUSSION
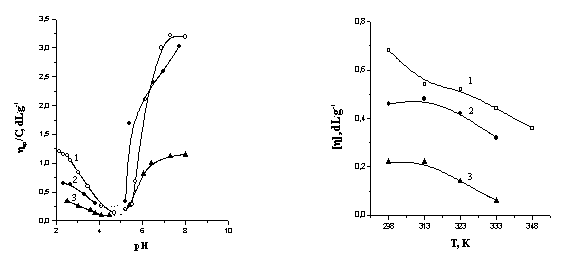
Fig.1 shows the
isoelectric points (pHIEP) of pure polyampholytes PA-I and PA-II
and their complexes with BSA and lysozyme. Both the PA-I and complexes
with composition of [MBSA]:[MPA-I]=2:5 and [Mlysozyme]:[MPA-I]=1:1
have the same pHIEP=5,0 and precipitate at this point. PA-II
and complexes with composition of [MBSA]:[MPA-II]=3:2 and
[MLysozyme]:[MPA-I]=1:1 have the values of pHIEP
that correspond to 8,6; 8,6 and 8,9, respectively. They are soluble at the
range of pH = 2-12. The different isoionic points of BSA and lysozyme suggests
that in order to bind proteins the charge of polyampholytes should be arranged
between pH=4,7 and 11,0. Therefore the conditions were selected such a manner
that lysozyme might interact only with PA-I via Coulombic interactions.
As seen from the curve of hsp/C –pH at pH=8,4
the PA-I is charged negatively (Fig.1) and interacts with positively charged
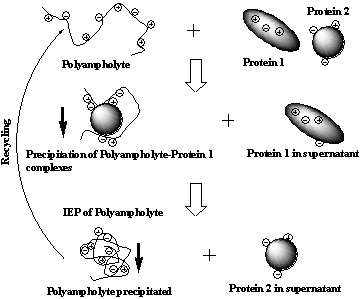
Lysozyme. In ternary BSA+Lysozyme+PA-I system the precipitation of PPC
was observed at pH=8,4. After centrifugation and separation of the
precipitate the intrinsic viscosity of supernatant was equal to [h]=0,07 dL/g. This
value coincides well with the intrinsic viscosity of pure BSA. Electrophoretic measurements also showed
that the supernatant contains only BSA. Thus in ternary system consisting of
BSA+Lysozyme+PA-I polyampholyte precipitates the Lysozyme. Adjustment of
solution pH to the IEP of pure PA-I (pHIEP = 5.0) leads to
precipitation of PA-I itself and detachment of Lysozyme (Scheme 1). Stability of soluble protein-PA-II
complexes was investigated with respect to temperature change and thermodynamic
quality of solvent (0,1 M KCl-ethanol mixture). Increasing of temperature up to
333 K decreases the intrinsic viscosities of both polyampholytes and PPC particles
(Fig.2). Such behavior of the system may be connected with compactization of
PPC particles due to strengthening of hydrophobic interactions in aqueous
solution. In 0,1 M KCl-ethanol mixture the protein-PA-II complexes are
soluble up to 50 vol.% of ethanol content. Further increase of ethanol content
resulted in precipitation of PPC.