Rutkovskaya D.S., Bashevaya T.S.
Donbas National Academy of Civil Engineering and
Architecture
Efficient use of resources in the
manufacture of phenol and acetone
Chemical and petrochemical industry is a source of raw materials for the production of consumer goods, especially chemical fibers and plastics. To obtain the phenol-formaldehyde resins that are used in the manufacture of plastics, phenol is used. Phenol is also processed into cyclohexanol, which is required for the synthetic fiber industry. The largest scale of production among all industries of polymeric materials evolves industry of plastics and synthetic resins. Continuously increasing demands of the chemical industry, as well as a number of other industries in phenols become more diverse [1; 2].
When
using as a feedstock with high content of aromatic hydrocarbons, the synthesis
of phenols can be represented in general form as the oxidation of hydrocarbons.
The method for the synthesis of cumene phenol obtained a wide circulation.
Feedstock for this process is benzene byproducts - acetophenone, methyl alcohol
and phenolic resin. Benzene is also a feedstock for many chemical manufactures;
therefore, an important task is the rational use of raw materials to improve
the environmental and economic indicators. To achieve a solution to such
problems, you may use different catalysts and activators of radical processes
[1; 3]. In this paper, as an activator is applied
dibenzo-18-crown-6-NaBr-N-gidroksiftalimid, the reaction of benzene peroxide
which runs the oxidation of cumene. This method can significantly improve the
efficiency of phenol synthesis through rational use of raw materials,
increasing the yield of the final product, which is especially important, as
currently all over the world 90% of phenol is synthesized by the method of
cumene.
Work objective: to improve production technology of phenol and acetone with the aim to conserve resources.
The method of
co-synthesis of phenol and acetone by cumene hydroperoxide has some obvious
advantages over other methods of synthesis of phenol.
When receiving phenol
through benzene it is necessary to spend large amounts of substances such as
sulfuric acid, caustic soda, sulfur dioxide; the equipment undergoes rapid
corrosion, the conditions of the individual stages of the process are very hard
[1].
Synthesis of phenol
with saponification of chlorobenzene associated with consumption of large
quantities of chlorine, caustic and hydrochloric acid. Using in the process of strongly
aggressive substances requires the use of expensive corrosion-resistant
materials. The disadvantage is also working under high pressure (up to 300
atm).
Raschig method is characterized
by the use in large quantities of hydrogen chloride, corrosion, harsh reaction
conditions and low conversion per pass, leading to high-energy costs.
Cumene method
compares favorably with these methods by mild conditions for carrying out all
stages of the process, using much smaller amounts of sulfuric acid and alkali,
the lack of chlorine and hydrochloric acid. Corrosion of equipment that occurs
in the alkylation, due to hydrolysis catalyst (aluminum chloride) and the
oxidation by forming a side benefit of formic acid is less intense and easier
to prevent than the corrosion of equipment when working on these methods [1;
4].
As a method of
production of acetone, the cumene method has several advantages compared with
others. Methods for the synthesis of acetone from propylene via isopropyl
alcohol include the manufacture of isopropyl alcohol, in obtaining of which the
sulfuric acid hydration of propylene you may apply and expose reduction the large
quantities of sulfuric acid. The conversion of isopropyl alcohol to acetone
takes place in harsh conditions with the use of expensive catalysts [5].
Direct methods for
synthesis of acetone – an oxidation of propane and butane mixes and propylene
oxidation by one-step method can compete with the receipt of acetone as a
byproduct in the synthesis of cumene phenol [2].
Feedstock in the
production of phenol by all the above-mentioned methods is benzene. The
greatest amount of benzene (about 1, 2 t) is consumed per 1 ton of phenol at chlorbenzene
method because, although the yield of phenol per chlorobenzene, and is 90-98%
of the theoretical, the degree of conversion of benzene into monochlorobenzene
is only 70-75%, and the rest of the benzene is chlorinated in more depth. When
working on other methods of 1 tonne of phenol consumes from 0.93 (at the sulfur
method) up to 0,95-1,05 t a benzene (at cumene method). In the production of
phenol by benzene, cumene method is consumed for formation of byproducts, the
number of which is larger than the number of by-products - benzene derivatives
- in the production of phenol by other methods (except chlorbenzene). The
feedstock is used not fully, when the output of useful product is 95% in each
of the three stages, the final output is only 86%. Reduction in the yield of
byproducts and waste (resin), and more complete utilization of raw materials at
the cumene synthesis method of phenol can significantly improve its efficiency
[5].
As a part of this study the optimal ratio of isopropylbenzene oxidation
rate and concentration of initiating system -
dibenzo-18-crown-6-NaBr-N-gidroksiftalimid to utilize fully the feedstock was
determined. To do this, you took different concentrations of the complex
dibenzo-18-crown-6-NaBr and on the laboratory facility determined the amount of
absorbed during the oxidation of isopropylbenzene oxygen depending on time.
Then, on the known data calculated the rate of oxidation of cumene and made a
comparative analysis of these characteristics at different concentrations of
the initiating system. The result of the study is the determination of the
optimum amount of the complex, which is a part of the initiating system, at a
concentration of which the most complete use of the feedstock, - benzene is
observed.
Five dependencies of the absorbed oxygen’s volume from time, under
different volume set of the initiating system were experimentally found. The
measurements were performed with a time interval of one minute for 38-50
minutes.
Based on the results we can conclude that the highest volume of oxygen is
consumed by the volume of the complex dibenzo-18-crown-6-NaBr 1 ml, ie at a
given concentration of the most active oxidation process proceeds
isopropylbenzene.
Figure 1 shows the rate of oxidation of cumene on the amount of the
complex dibenzo-18-crown-6-NaBr, which is a part of the initiating system.
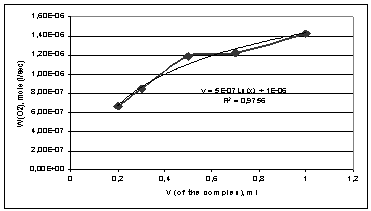
Figure 1 - Dependence of the rate of oxidation of cumene on the amount
of the complex dibenzo-18-crown-6-NaBr, which is a part of the initiating
system.
Analyzing this dependence we can argue that it is appropriate to cure the
oxidation process at the content of the initiation complex of 0,5 - 1 ml per 1 ml
of cumene. Reducing the amount of the initiation complex 0,5 to 0,3 ml leads to
a decrease in the oxidation rate in 1,5 times. The most acceptable is the
amount of the complex dibenzo-18-crown-6-NaBr equal to 1 ml.
According to the results of the research data, the optimal parameters of
the joint production of phenol and acetone, this allows the maximum use of raw
materials with a maximum output of phenol. These parameters are the contents of
the complex dibenzo-18-crown-6-NaBr on the initiating system, equal to 1 ml and
the rate of oxidation of cumene is equal to 1,43∙10-6 mol/L∙sec. At
a given concentration of initiating system, the consumption of raw materials in
the production of phenol was reduced from 0.95 tons of benzene per 1 tonne of
phenol up to 0.88 t.
References:
1.
Кружалов Б.Д. Совместное
получение фенола и ацетона. / Б.Д. Кружалов, Б.Н. Голованенко. – M. : Химия, 1963. – 200 с.
2.
Лебедев
Н.Н. Химия и технология основного органического и нефтехимического синтеза.
/ Н.Н. Лебедев. – М. : Химия, 1989. – 608 с.
3. Петров А.А. Органическая химия. / Петров А.А., Бальян Х.В., Трощенко А.Т. – М. : ВШ., 1969. – 306 с.
4.
Харлампович Г.Д. Фенолы. / Харлампович Г.Д., Чуркин Ю.В. – М. : Химия,
1986. – 375 с.
5.
Кондауров Б.П. Общая химическая технология. / Б.П. Кондауров, В.И.
Александров, А.В. Артемов. – М. : Издательский центр «Академия», 2005. – 336 с.