Prooxidant and
Antioxidant Action of Psoralens
Teresa Michalska
Institute of Physics, Szczecin University of
Technology
Al. Piastow 48/49, 70-310
Szczecin, Poland
ABSTRACT: The effect of
psoralens (psoralen, 5-methoxypsoralen, 8-methoxypsoralen, khellin and
visnagin) on a chemical system involving a superoxide radical was tested using
the chemiluminescence method.
High doses of psoralens (1mM) showed
prooxidative effects. Incubation of psoralens at lower doses showed khellin
(0.8 mM), 8-methoxypsoralen (0.1 mM), visnagin (0.05mM) and psoralen (0.03 mM)
have antioxidant effects.
KEYWORDS:
psoralens; chemiluminescence; superoxide radicals.
1. Introduction
Psoralens (PSOs) or furanocumarins are well known as photoreactive
compounds [1].The compounds are also
very often used in dermatology for the photochemotherapy of several diseases
such as vitiligo, psoriasis, atopic eczema and
others [2,3].The combination of PSOs usage with UV-A irradiation is
known as PUVA therapy [4]. It has been shown that UV-A irradiation of PSOs in
the air atmosphere generates a large amount of photoproducts, known as reactive
oxygen species (ROS) such as: superoxide radical anion (![]() ), hydroxyl radical (
), hydroxyl radical (![]() ), hydrogen peroxide (
), hydrogen peroxide (![]() ) and singlet oxygen (
) and singlet oxygen (![]() ) [5-8]. However, it has been observed that PSOs at high
doses also generates ROS in PUVA inducing undesirable site effects (e.g. skin
cancer, edena, skin erythema) [6,10,11].
) [5-8]. However, it has been observed that PSOs at high
doses also generates ROS in PUVA inducing undesirable site effects (e.g. skin
cancer, edena, skin erythema) [6,10,11].
This paper deals with experiments concerning the
effect of five PSOs: psoralen (PSO), 5-methoxypsoralen (5-MOP),
8-methoxypsoralen (8-MOP), khellin (KHL) and visnagin (VIG) on the light
emission from a chemical system generating ![]() . These compounds (see structures, Fig. 1) are hypothesized
to have anti-oxidative and pro-oxidative properties. The properties may be
examined using the chemiluminescent technique, which is the sensitive
analytical method for
. These compounds (see structures, Fig. 1) are hypothesized
to have anti-oxidative and pro-oxidative properties. The properties may be
examined using the chemiluminescent technique, which is the sensitive
analytical method for ![]() detection, especially
for the evaluation of the redox property of a
detection, especially
for the evaluation of the redox property of a
compound.
Fig.1.
The chemical structure of PSOs.
2.
Materials and Methods
PSOs,
superoxide dismutase (SOD, EC1.15.11, 5600 U/mg) and catalase (19,900 U/mg from
bovine liver thymol free) were purchased from Sigma Chemical Co. (st.Louis,
MO). Potassium superoxide (KO2) were from Fluka, Buchs
(Switzeralnd). Dimethyl sulfoxide (DMSO) was obtained from Aldrich Chemical Co.
(Milwaukee. WI). Crown ether (18-crown-6), Tiron
(1,2-dihydroxybenzene-3,5-disulfonic acid), trolox
(6-hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid),
5,5-dimethyl-cyclohexanodion-1,3 (DMCH) and compounds that were used as
antioxidant were purchased from Merck (Darmstadt, Germany).
Superoxide
anion radical (![]() ) was prepared according to the following procedure: 60mg of
18-crown-6 was dissolved in 10 mL of dry DMSO and then 7 mg of KO2
was added quickly to avoid contract with air humidity [12]. The mixture was
stirred with a magnetic stirrer for 1 h to give a pole yellow solution of 10 mM
) was prepared according to the following procedure: 60mg of
18-crown-6 was dissolved in 10 mL of dry DMSO and then 7 mg of KO2
was added quickly to avoid contract with air humidity [12]. The mixture was
stirred with a magnetic stirrer for 1 h to give a pole yellow solution of 10 mM
![]() which was at least 1 h
stable at room temperature. DMSO mixtures of PSOs were prepared at room
temperature and kept in the dark.
which was at least 1 h
stable at room temperature. DMSO mixtures of PSOs were prepared at room
temperature and kept in the dark.
The influence of PSOs on the system generating ![]() was detected by a chemiluminescent technique. The
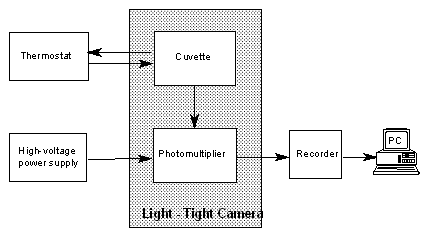
chemiluminescence (CL) intensity was recorded with a set especially designed
and constructed in Institute of Physics, Szczecin University of Technology
(Fig. 2). The basic instrumentation consists of a specially designed chamber,
containing glass cuvette with 50 mm diameter, placed in light-tight box. The
cuvette was exhausted and washed using a B-169 vacuum system (Büchi,
Flawill Switzerland). The photomultiplier type EMI 9553Q with a S20 cathode,
sensitive in the range 200-800 nm, interfaced with a personal computer for date
acquisition and handling was used as a detector.
was detected by a chemiluminescent technique. The
chemiluminescence (CL) intensity was recorded with a set especially designed
and constructed in Institute of Physics, Szczecin University of Technology
(Fig. 2). The basic instrumentation consists of a specially designed chamber,
containing glass cuvette with 50 mm diameter, placed in light-tight box. The
cuvette was exhausted and washed using a B-169 vacuum system (Büchi,
Flawill Switzerland). The photomultiplier type EMI 9553Q with a S20 cathode,
sensitive in the range 200-800 nm, interfaced with a personal computer for date
acquisition and handling was used as a detector.
The
apparatus used provides the opportunity for simultaneously calculation of the
light sum, i.e., area (SI) under the kinetic curve, I = f(t) within any chosen
time interval (![]() ).
).
All
data are presented as a mean ± SD of at least six different experiments. P-values
< 0.05 were considered as statistically significant.
Fig.2. Block diagram of chemiluminescence measurements
system.
3. Results
and discussion
Reactivity
of PSOs
with ![]() was monitored using
the
was monitored using
the ![]() radical at a
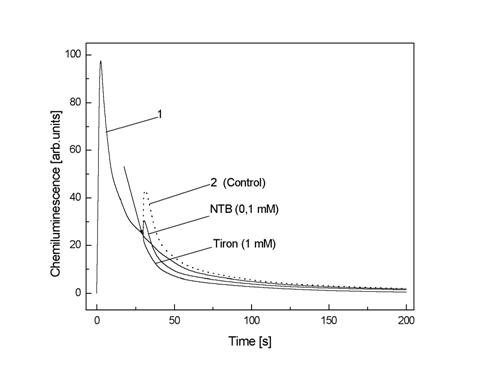
concentration of 1 mM in DMSO. The mixture elicits a strong CL, (Fig. 3, curves
1). The tested PSOs were added 30s after the start reaction and SI were detected during 60s. An addition of DMSO alone
at the same concentration as in the PSOs/DMSO solution resulted in “flash”
followed by a increase in CL/Fig. 3, curve 2). An area under curve 2 considered
as the control sum(
radical at a
concentration of 1 mM in DMSO. The mixture elicits a strong CL, (Fig. 3, curves
1). The tested PSOs were added 30s after the start reaction and SI were detected during 60s. An addition of DMSO alone
at the same concentration as in the PSOs/DMSO solution resulted in “flash”
followed by a increase in CL/Fig. 3, curve 2). An area under curve 2 considered
as the control sum(![]() ). The quenching ratio was defined as
). The quenching ratio was defined as ![]() , where
, where ![]() represents the light
sum without a inhibitor,
represents the light
sum without a inhibitor, ![]() represents the light
sum with a inhibitor.
represents the light
sum with a inhibitor.
Fig.3. The effect of DMSO, Tiron and NTB on
chemiluminescence intensity from 1 mM ![]() generated in DMSO. The
arrow indicate the moment of reagent addition
generated in DMSO. The
arrow indicate the moment of reagent addition
Oostehuizen et al.
[13] reported have disproportionation
of ![]() in the system with KO2
generating these radicals:
in the system with KO2
generating these radicals:
![]() +
+ ![]() + 2H+
+ 2H+ ![]() H2O2
+
H2O2
+ ![]() (1)
(1)
This reaction is accompanied by generation of small amounts of ![]() [14]. An interaction
between
[14]. An interaction
between ![]() . and H2O2 in the presence of a
reducing agent such as DMSO can lead to the
. and H2O2 in the presence of a
reducing agent such as DMSO can lead to the ![]() formation [15]
formation [15]
![]() + H2O2
+ H2O2 ![]()
![]() + HO- +
+ HO- + ![]() (2)
(2)
Another possible reaction responsible for the ![]() generation under our
experimental conditions is on interaction of
generation under our
experimental conditions is on interaction of ![]() and
and ![]() radical as follows:
radical as follows:
![]() +
+ ![]()
![]() HO-
+
HO-
+ ![]() (3)
(3)
as well as disproportionation of H2O2
H2O2 +
H2O2 ![]() 2H2O +
2H2O + ![]() (4)
(4)
The generation of ROS in the examined systems was confirmed by observed
quenching effect on the CL of inhibitors specific for ![]() ,
, ![]() , H2O2 and scavengers of 1O2 [16] (Table 1).
, H2O2 and scavengers of 1O2 [16] (Table 1).
Among the tested inhibitors Tiron and NTB appeared to be the strongest
quenchers of the light emission. In contrast, an addition of SOD (0.1mg/mL)
know inhibitor of ![]() to the reaction system
increased the light of emission (quenching ratio was about -41 %). The observed
increase in the CL in the presence of SOD in study may be due to the fact that
SOD catalyses the formation of ROS in the presence of H2O2
and reducing agent [17,18].
to the reaction system
increased the light of emission (quenching ratio was about -41 %). The observed
increase in the CL in the presence of SOD in study may be due to the fact that
SOD catalyses the formation of ROS in the presence of H2O2
and reducing agent [17,18].
Also the aqueous solution of histidine, DMCH (quenchers of 1O2)
and catalyse (enzyme responsible for destruction of H2O2
) the CL by 42%, 36% and 48% respectively.
However, water alone at the same amount (5%) as the tested antioxidant /
H2O solution decreased the CL by about 10%
Table 1. Effect of inhibitors ROS on chemiluminescence from 1
mM ![]() generated in DMSO.
generated in DMSO.
|
Compound |
Concentration |
Quenching % |
|
NBT |
1 mM |
67 ± 6 |
|
Tiron |
1 mM |
58 ± 5 |
|
Trolox |
1 mM |
36 ± 5 |
|
SOD |
100 mg/mL |
-41 ± 4 |
|
SOD |
50 mg/mL |
-17 ± 3 |
|
Catalase* |
200 mg/mL |
48 ± 6 |
|
Catalase* |
50 mg/mL |
16 ± 2 |
|
Histidine* |
1 mM |
42 ± 4 |
|
5,5-dimethylocykloheksanodione-1,3 (DMCH)* |
0,5 mM |
36 ± 4 |
|
Thiourea |
1 mM |
28 ± 3 |
|
Ethanol |
1 M |
32 ± 4 |
|
Mannitol |
1 mM |
39 ± 5 |
The compounds were dissolved in
DMSO.
* - the compounds dissolved in water.
An addition of thiourea, ethanol, or
mannitol (efficient scavenger ![]() ) caused a decrease in CL of about 28%, 32% and 39%
respectively.
) caused a decrease in CL of about 28%, 32% and 39%
respectively.
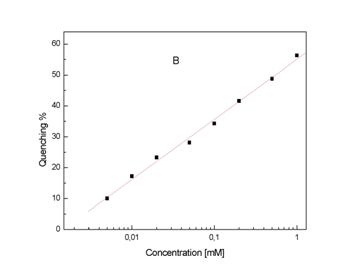
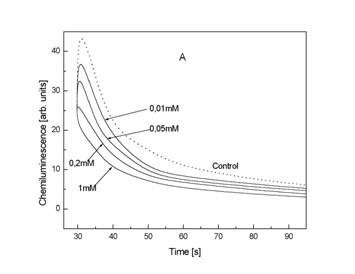 Fig.4. The effect of different concentration of Tiron on CL from 1
mM
Fig.4. The effect of different concentration of Tiron on CL from 1
mM ![]() generated in DMSO (A).
Relationship between quenching ratio and concentration Tiron (B).Details are reported under ‘Materials and Method’.
generated in DMSO (A).
Relationship between quenching ratio and concentration Tiron (B).Details are reported under ‘Materials and Method’.
Figure 4A presents quenching of CL in the presence of different
concentration of Tiron. Final results were calculated with
a help of regression equation and are presented as quenching ratio Qx
= a + b Cx where Cx the logarithmics concentration of
Tiron (Fig 4B). Regression coefficients for Tiron are: a = 55,05 ±
0.79 and a value for the slope b = 19.44 ±0.57 (mean ±
SD); multiple correlation coefficient r = 0.997. A very similar effect presents KHL in a range
0,8 – 0.002 mM (Fig. 5A). Regression coefficients for KHL are: a = 32,85 ±
0.59 and b = 11,06 ± 0.39; multiple correlation
coefficient r = 0.996.
A set experiments was performed to determine the reactivity of PSOs:
psoralen, 5-methoxypsoralen, 8-methoxypsoralen, khellin and visnagin towards ![]() using the CL
technique. The decrease or increase in the light emission from the system
generating
using the CL
technique. The decrease or increase in the light emission from the system
generating ![]() was dependent on the
concentration added PSO and their kind (Fig 5).
was dependent on the
concentration added PSO and their kind (Fig 5).
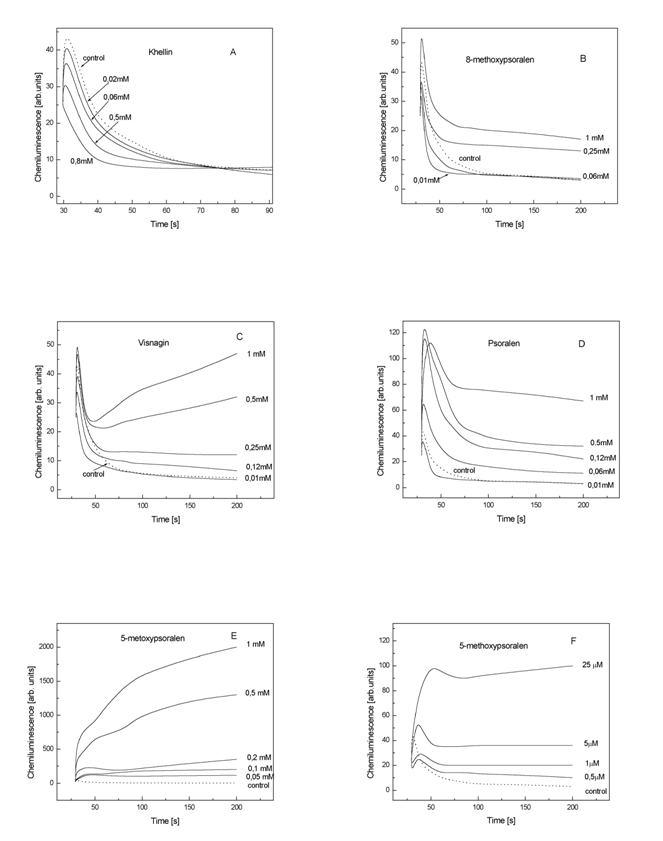
Fig.5. The effect of psoralens concentration on chemiluminescence
intensity from 1 mM ![]() generated in DMSO.
generated in DMSO.
The data demonstrate that all tested PSOs in doses of 1 mM increased the
CL (Table 2), thus showing prooxidative effects; however the enhancing effects
exerted by KHL were very small in comparison with 5-metoxypsoralen. Incubation
of PSOs at lower doses with the ![]() generating system
showed that KHL (0.8 mM), 8-MOP (0.1
mM), VIG (0.05 mM) and PSO (0.03 mM)
exerted quenching effect on the light emission. In contrast 5-MOP at that
concentration 0.5 mM did not exert the quenching effects (Fig 5F).
generating system
showed that KHL (0.8 mM), 8-MOP (0.1
mM), VIG (0.05 mM) and PSO (0.03 mM)
exerted quenching effect on the light emission. In contrast 5-MOP at that
concentration 0.5 mM did not exert the quenching effects (Fig 5F).
Table 2. Effect of 1 mM Psoralens on chemiluminescence from 1
mM ![]() generated in DMSO
generated in DMSO
|
Psoralens |
Enhancement, R %
|
|
R 60 s |
R 120 s |
|
|
5-metoxypsoralen |
2861 ± 10 |
4522 ± 15 |
|
Psoralen |
440 ± 5 |
633 ± 6 |
|
Visnagin |
104 ± 4 |
226 ± 5 |
|
8-metoxypsoralen |
68 ± 3 |
127 ± 4 |
|
Khellin |
-37 ± 2 |
1,2 ± 0,3 |
The enhancement R was calculated
using by ![]() , where
, where ![]() is the light sum with
a PSO and
is the light sum with
a PSO and ![]() is the light sum without a PSO. R60s, R120s
– light sum detected during 60s and 120s, respectively.
is the light sum without a PSO. R60s, R120s
– light sum detected during 60s and 120s, respectively.
The chemiluminescence sums increased in the order KHL < 8-MPO <
VIG < PSP < 5-MOP, indicating increased production of ROS. We observed a
very similar sequence of the PSOs behavior as enhancers of ![]() formation via Fenton
formation via Fenton![]() reaction in dark in experiments using reducion of
ferricytochrome c by
reaction in dark in experiments using reducion of
ferricytochrome c by ![]() [9].
[9].
4. Conclusion
The chemiluminescence method is a simple and convenient technique to examine the PSOs redox properties at ambient temperature.
Incubation of tested PSOs in the system producing light emission showed that
khellin, 8-methoxypsoralen, visnagin and psoralen at low concentration show
antioxidative effect in the system generating superoxide anion radical. When
high doses of these compounds had been used they showed prooxidant property.
5.References
1. Kitamura,
N.; Kohtani, S.; Nakagaki, R. J.Photochem Photobiol C 2005, (6), 168-185.
2. Lehr, G.J.; Barry, T.L.; Franolic, J.D.; Petzinger, G. J.
Pharm. Biomed. Anal. 2003, (33), 627-637.
3. Edelsonb,
R.L. J.Photochem Photobiol 1991, (B10), 165-171.
4. Cardoso, C.A.; Vilegas, W.; Honda, N.K. J.Pharm. Biomed. Anal.
2000, (2), 203-214.
5. Liu, Z.; Lu, Y.; Lebwohl, M.; Wei, H. Free Rad Biol Med 1999,
(20), 751-756.
6. Foote, G.S. Photochem Photobiol 1990, (54), 659-668.
7. Potapienko,
A. J Photochem Photobiol 1992, (B9), 1-33.
8. Chouchi,
D.; Barth, B. J Chromatogr 1994,(A 672), 177-183.
9. Aboul-Enein,
H.Y.; Kladna, A.; Kruk, I.; Lichszteld, K.; Michalska T. Biopolymers
(Biospectroscopy) 2003,(72), 59-68.
10. Pathak, M.A.; Joshim, P.C. Biochim Biophys Acta 1984,(798),
115-126.
11. Halliwell, B.; Gutteridge, J.M.C. Methods Enzymol 1990, (186),
1-85.
12.
Valentine,
J.S.; Miksztal, A.R.; Sawyer, D.T. Methods Enzymol. 1984,(105), 71-80.
13.
Oostehuizen,
M.M.J.; Engelbercht, M.E. ;Lambrechts, H.;Greyling,D.;Levy, R.D. J.Biolumin
Chemilumin 1977(12) 277-284.
14.
Krynsky,
N.I. TIBS 1997,(2), 35-38.
15.
Haber, F.;
Weiss, J.J. Proc R Soc London Ser.A 1934,(147),332-351.
16.
Kruk, I.
Environmental Toxicology and Chemistry
of Oxygen Species. The Handbook of
Environmental Chemistry. Reactions and Processes Part 1
Hutzinger, O; Kruk, I. (eds). Springer-Verlag Berlin 1998.
17.
Auroma,
O.I. Free Radic Biol Med. 1996,(20),675-705.
18.
Roe,J.M.;
Wiedan-Pazos, M.; Moy,V.N. et al. Free Radic Biol Med. 2002,(32),169-174.