Chemistry
Nadirov
R.K.1, Dossymkhanova M.K.2
1Kazakh National University named after al-Farabi,
Almaty, Kazakhstan
2Central Kazakhstan institute of technology and management,
Balkhash, Kazakhstan
ELECTROSYNTHESIS
OF FLAVOPIRIDOL
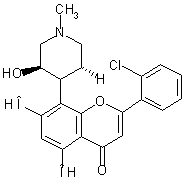
Flavopiridol
((-)-2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3R,4S)-3-hydroxy-1-methyl-4-piperidinyl]-4H-1-benzopyran-4-one)
(see Fig. below) is a synthetic flavon with antitumor activity [1-3]. Potent
inhibitor of cyclin-dependent kinases (CDKs). Induces apoptosis in
certain tumor cells. Interacts with multidrug resistance protein 1 (MRP1). Soluble
in chloroform; slightly soluble in water. For the first time synthesized
at the end of past century.
|
|
Known processes
of synthesis of flavopiridol are difficult and sequential. The present work
has led to the development of a facile and environmentally friendly electrochemical method for synthesis of
flavopiridol on the base of chrysin (5,7-dihydroxyflavone) and N-methyl
piperidine. All of reagents were
reagent grade materials from
Aldrich. |
The first stage of the process
of synthesis of flavopiridol is chlorination of chrysin. Electrolysis is
performed in an electrolytic cell equipped a diaphragm to separate the cell
into a cathode compartment and anode compartment. The cell is placed in a bath
of ice water. The anode is a 5 cm2 plate of glassy carbon and the
cathode is stainless stell. The anode compartment is filled with 200 ml of
mixed solvent, 0.5g of KCl and 60 mg of chrysin. The cathode compartment is
filled with 100 ml of mixed solvent
and 0.3 g of KCl. Electrolysis were carried out with a constant potential (1.0
V versus Ag+/Ag).
Electrolysis of N-methyl piperidine is performed in
other divided cells at room temperature in the mixed solvent in the
presence of KOH. The anode compartment contains 10 ml of N-methyl piperidine and 50 ml of mixed
solvent and 0.2 g of KOH. The working electrode (anode) was a glassy carbon
plate (1 ×1.5 cm ). Electrolysis were carried out with a constant current
density (25 mA/cm2). Electrolysis is stopped after passing through
the cell 1.5 F per mole of N-methyl piperidine.
The anolyte of electrolysis of N-methyl piperidine were added into the
anode compartment of cell of electrolysis of chrysin when the current decreased
by more than 70%. Electrolysis is stopped after passing through the cell 3 F
per mole of chrysin.
At the end of electrolysis the
solvent of anolyte were vacuum
distillated and the residuum were washed with aqueous NaHCO3 and
water and purified with column chromatography (silica
gel). After purification,
the products were characterized by IR and 1H NMR.
According
to the IR and 1H NMR results we suggest that flavopiridol (yellow
powder) were synthesized (yield 3%).
We
suggest that the first stage of the process of synthesis of flavopiridol is
chlorination of chrysin; one of products of this stage is 2/-chlorchrysin.
Determination
of the next stages will be thanks to prospected investigations using electrochemical (voltammetry) and
physicochemical methods. We suppose the creating favourable conditions for
selective synthesis of 2/-chlorchrysin is necessary condition for
increasing of desired product yields. It may be made by optimization of
experiment by variation of next parameters: concentration of substrate; concentration of KCl; time of electrolysis; pH. The next
stage of process is synthesis of flavopiridol on base of 2/-chlorchrysin
and N-methyl piperidine.
References
1.
Flavopiridol induces G1 arrest with
inhibition of cyclin-dependent kinase (CDK)2 and CDK4 in human
breast carcinoma cells: B.A. Carlson, et al.; Cancer Res. 56, 2973
(1996)
2.
Flavopiridol induces apoptosis in chronic
lymphocytic leukemia cells via activation of caspase-3 without evidence of
bcl-2 modulation or dependence on functional. p53: J.C. Byrd, et al.; Blood 92,
3804 (1998)
3.
Flavopiridol synergizes TRAIL cytotoxicity by
downregulation of FLIPL: T.E. Fandy, et al.; Cancer Chemother. Pharmacol. 60, 313 (2007)