Hodyna D., Trush M., Metelytsia L., Tarasyuk O., Rogalsky S.
Institute of Bioorganic Chemistry and
Petrochemistry, National Academy of Science of Ukraine
Murmanska St., 1, Kyiv 02094, Ukraine
A
comparative study of imidazolium and imidazolinium ionic liquids: antimicrobial
activity and acute toxicity
Ionic
liquids (ILs) are low temperature molten salts composed of bulky organic
cations and various anions. Due to unusual combination of extremely low vapour
pressure, high thermal stability and ionic conductivity ILs have found numerous applications such as alternative “green” solvents in organic
synthesis, polymer chemistry, enzyme catalysis, drug delivery systems etc. [1].
Recently imidazolium ILs comprising bulky 1,3-dialkylimidazolium cations with
long aliphatic chains were found to possess broad range of
antimicrobial activity against both Gram-negative and Gram-positive bacteria,
fungi and algae, as well as pronounced anti-biofilm activity [2-4]. It was indicated that alkyl substituted imidazolium
cations interact with the negatively charged bacterial cell membrane and
disrupt it, causing leakage of cell. Due to their lipophilic nature, the alkyl
chains of imidazolium cations may interact with the cell membrane constituents,
thereby increasing membrane permeability [2].
However, potential toxicity of ILs is one of the key
factors determining their availability for practical applications, especially
in medical devices, protective coatings etc. From this point of
view, water insoluble ILs can be very promising antimicrobial
additives due to their high washing resistance and therefore potentially higher
durability, as well as lower environmental impact.
The aim of this research was to evaluate antimicrobial activity and
acute toxicity of two water immiscible ILs comprising tetrafluoroborate anion
and such cations as 1-dodecyl-3-methylimidazolium (DMIM-BF4) and
2-dodecylamino-imidazolinium (DAIM-BF4). It should be noted that
there are no data in the literature concerning the biological activity of
imidazolinium based ILs.
ILs
were synthesized according to following schemes:
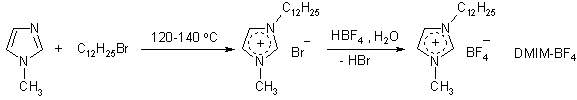
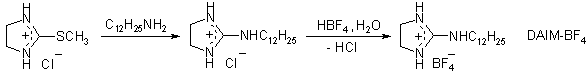
Antimicrobial properties of the ILs were tested by using standard agar disk
diffusion method [5]. The tested
bacterial strains were Staphylococcus aureus (ATCC-25923), Escherichia coli (ATCC-25922), Pseudomonas aeruginosa
(ATCC-27853) and fungi strains were Candida albicans (M 885
ATCC 10231) and clinical
isolates Candida albicans, Candida glabrata and Candida krusei.
Bacterial strains were subcultured on Mueller Hinton Agar and fungal
strains were subcultured on Sabouraud agar
in Petri plates according to the
manufacturer’s instructions. All ILs were investigated
in concentrations of 1.0; 0.1 % and were dissolved in DMSO. 0,02 ml of test compounds were applied on the sterile paper discs (6 mm diameter). The
microbial culture was evenly poured onto the surface of agar plates into a
volume of 0,2 ml of sterile saline solution to produce final concentration of
1∙105 CFU /ml. The plates were incubated at +37 ° C for 24 hr.
The antimicrobial activity of ILs was assessed by measuring zone diameter
of the growth inhibition (in mm).
Acute toxicity testing was carried out on Zebrafish (Danio rerio) as an
aquatic model. The maximum allowable concentration
of ILs (LD50) was taken
5.5 mg/l [6].
The results of
antimicrobial activity of ILs are presented
in Table. 1
Table 1. Antimicrobial activity of DAIM-BF4 and
DMIM-BF4 by zone diameters
of growth inhibition of tested
bacterial and fungal strains
|
¹ |
Tested microorganisms |
Conc., % |
Inhibition zone of ILs, mm |
|
|
DAIM-BF4 |
DMIM-BF4 |
|||
|
1 |
Å. coli ATCC |
1,0 |
35 |
22 |
|
0,1 |
20 |
10 |
||
|
2 |
St. aureus ATCC |
1,0 |
24 |
32 |
|
0,1 |
20 |
23 |
||
|
3 |
Ps. aeruginosa ATCC |
1,0 |
21 |
30 |
|
0,1 |
16 |
21 |
||
|
4 |
C. albicans ATCC |
1,0 |
30 |
30 |
|
0,1 |
17 |
16 |
||
|
5 |
C. albicans |
1,0 |
25 |
28 |
|
0,1 |
13 |
22 |
||
|
6 |
C. glabrata |
1,0 |
>40 |
30 |
|
0,1 |
25 |
20 |
||
|
7 |
C. krusei |
1,0 |
>40 |
>40 |
|
0,1 |
30 |
22 |
||
According to the obtained data both ILs have high antimicrobial activity, showing
broad inhibition zones for both bacterial and fungi test-cultures. It can be suggested
that as in the case for imidazolium ILs, the antimicrobial properties of DAIM-BF4
are also caused by the presence of imidazolinium cation with delocalized charge,
as well as hydrophobic alkyl tail.
The investigation of acute toxicity (LD50) of ILs on the model hidrobiont Zebrafish (Danio rerio) showed that DAIM-BF4 is three
times less toxic (LD50 was 20 mg/l) than DMIM-BF4 (LD50
was 7 mg/l). Figure 1 and Figure 2 presents summary data for antimicrobial activity and acute toxicity of the
investigated ILs.
Thus, the results of our research indicate on availability of
imidazolinium salts as potential antimicrobial agents with broad range of
biological activity and reduced toxicity.
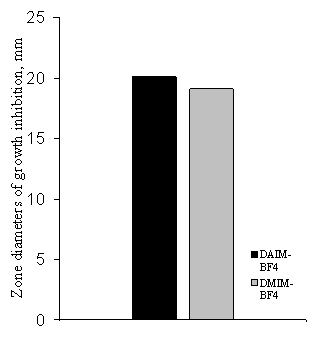
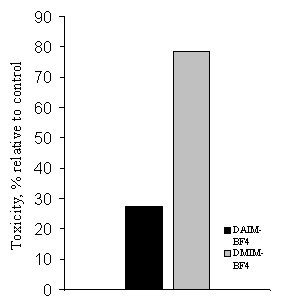 Figure 1. Antimicrobial
activity of ILs Figure 2. Acute toxicity
of ILs
Figure 1. Antimicrobial
activity of ILs Figure 2. Acute toxicity
of ILs
References
1. Welton, T. (1999) Room-Temperature Ionic
Liquids. Solvents for Synthesis and Catalysis. Chem Rev 99 (8),
2071-2084.
2. Pernak, J., Sobaszkiewicz, K., Mirska, I.
(2003) Anti-microbial activities of ionic liquids. Green Chem 5, 52-56.
3. Docherty, K., Kulpa, C. (2005) Toxicity and antimicrobial
activity of imidazolium and pyridinium ionic liquids. Green Chem
7, 185-189.
4. Carson, L., Chau, P., Earle, M., Gilea,
M., Gilmore, B., Gorman, S., McCann, M., Seddon, K. (2009) Antibiofilm
activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem 11, 492–497.
5. Bauer A.,
Kirby W.,
Sherris J.,
Turck M.
Antibiotic susceptibility testing by a standardized single disk method // Am. J. Clin. Pathol. - 1966. - 45, No 4. - P.
493-496.
6. Øàëÿïèí Ã. Èññëåäîâàíèå äåéñòâèÿ áèîöèäîâ (íà ïðèìåðå
ïîëèãåêñàìåòèëåíãóàíèäèíîâ) íà ãèäðîáèîíòîâ ðàçëè÷íûõ ñèñòåìàòè÷åñêèõ ãðóïï //
Ðûáîõîçÿéñòâåííàÿ òîêñèêîëîãèÿ. - 2010. - 11, ¹1 (41). - ñ. 199-206.