Zakharenko O.K., Trusova
V.M., Gorbenko G.P.
HEMOGLOBIN BINDING
TO MODEL MEMBRANES
Elucidating the nature of hemoglobin (Hb) –
lipid interactions seems to be of great importance in several main aspects.
First, Hb-lipid systems provide a useful model for gaining
insight into the mechanisms by which soluble proteins interact with biomembranes. Second, liposome-encapsulated Hb has found potential use as a red blood cell substitute.
Third, Hb electrocatalytic
and peroxidase activities are widely exploited in
biosensing. In the present work we used several spectroscopic techniques to
characterize lipid-associating and membrane-modifying properties of Hb. More specifically, our attention was focused on: i) Hb adsorption onto lipid
bilayer, ii) protein conformational changes upon complexation
with model membranes, and iii) Hb effect on
physicochemical properties of lipid bilayer.


Fig. 1. (A) Elution profile of Hb-liposome mixture obtained by size-exclusion
chromatography on Toyopearl HW-
The first step of the study involves obtaining the adsorption isotherms
by separation of Hb-liposome complexes and free Hb using gel filtration technique (Fig. 1). Lipid
vesicles were prepared from zwitterionic lipid phosphatidylcholine (PC) and
anionic lipid cardiolipin (CL) with CL content 5, 10 and 20 mol%.
Hb complexes
with lipids are stabilized by electrostatic and hydrophobic interactions, the
latter favors protein penetration into the nonpolar
region of membrane. The obtained binding data were quantitatively analyzed in
terms of lattice model of large ligand adsorption to membranes allowing for the
possibility of protein insertion into bilayer interior. Presented in Table 1
are thermodynamic parameters of Hb-lipid binding –
association constant (Ka),
free energy change (ΔG), number
of lipid molecules per bound protein (n).
Table
1
Thermodynamic parameters of Hb-lipid binding
|
System |
n |
Ka,
M-1 |
ΔG, kJ/mol |
|
PC
|
18.8±5.6 |
(1.8±0.5)×104 |
-24.3±7.3 |
|
PC:CL
(5 mol% CL) |
16.6±5.0 |
(2.4±0.7)×103 |
-19.3±5.8 |
|
PC:CL
(10 mol% CL) |
19.1±5.7 |
(4.5±1.4)×103 |
-20.8±6.2 |
|
PC:CL
(20 mol% CL) |
17.3±5.2 |
(6.7±2.0)×103 |
-21.8±6.5 |
The
highest Hb affinity was found for neutral PC
liposomes, suggesting the predominant role of hydrophobic binding component. CL-containing
systems were featured by the lower association constants, although increasing
with CL content.
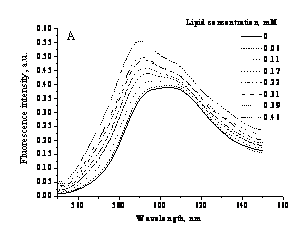
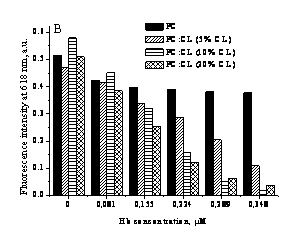
Fig. 2. (A) Fluorescence
spectra of rhodamine
At the second step of the
study we evaluated the possibility of employing rhodamine
101 (R101) as a specific fluorescent probe for detecting Hb
conformational changes. The spectral parameters of R101 remained virtually
unchanged in the presence of liposomes, suggesting that this probe either is
insensitive to its transition from the aqueous to lipid phase or is incapable
of associating with lipid bilayer. However, it appeared that R101 can easily
associate with Hb as can be judged from R101
fluorescence intensity changes. Addition of isolated Hb-liposome
complexes to R101 in buffer was accompanied by the changes in the shape of emission spectra
suggesting the existence of several spectral bands and enhancement of
shorter-wavelength (590 nm) component with increasing amount of membrane-bound Hb (Fig. 2A). These effects are most likely to arise from Hb structural changes at lipid-water interface.
At the last step of the study fluorescent
probe DSP-12 was employed to obtain information about Hb
effect on physicochemical properties of lipid bilayer. Hb-lipid
binding was accompanied by the decrease of DSP-12 fluorescence intensity, with the
magnitude of this effect being increased with CL content. Suppression of this
effect in the presence of free radical scavenger thiourea
allowed us to conclude that DSP-12 is sensitive to Hb-induced
lipid peroxidation. Deconvolution
of DSP-12 fluorescence spectra yielded two spectral components with emission
maxima around 567 and 620 nm, which correspond to the probe populations
differing in the location with respect to lipid-water interface. Relative
contribution of these components proved to depend on Hb
concentration and lipid composition of model membranes. More specifically, contribution
of the shorter-wavelength component increases with CL content and Hb amount. These findings suggest that fluorescence of the
longer-wavelength component adopting more shallow bilayer location is quenched
by the polar products of free radical reactions, accumulating in the
interfacial region.
Cumulatively, the present study
revealed several new features of Hb-lipid
interactions which may prove of interest both from fundamental and practical
viewpoints.