A.V.
Yudintsev1, V.M. Trusova1, G.P. Gorbenko1, T.
Deligeorgiev2,
A.
Vasilev2,
1V.N. Karazin Kharkov
National University, 4 Svobody Sq., Kharkov,
61077
2Department of
Applied Organic Chemistry, Faculty of Chemistry, University
of Sofia, Bulgaria
pre-formulation studies of potential anticancer drug – Eu(III)
coordination complex
Development of new classes of pharmaceuticals
with the improved properties is one of the leading areas of biomedical
research. However, appropriate delivery of drugs to their sites of action is
complicated by a number of factors, particularly, by poor translocation of the
drug through cellular membrane which represents a major barrier for
biopharmaceuticals. The efficacy of
many drugs is improved by the development of their liposomal formulations. Liposome
delivery systems can enhance drug solubility, reduce toxicity and improve
stability of the drug by its protection from chemical degradation or
transformation. Liposomal formulations have been
produced for a number of anticancer drugs (doxorubicin, daunorubicin,
cytosine arabinoside, etc.). Though, it is
known that the physicochemical characteristics of liposomes, including lipid
composition, size, membrane fluidity, surface properties may exert influence on
the rate of blood clearance and nature of tissue distribution of a drug. For
this reason, while using liposomes as delivery systems for lypophilic
drugs, it is necessary to know the character of a drug effect on the structure
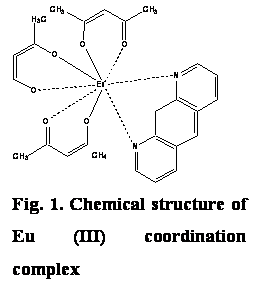
and properties of a lipid bilayer. In the present work we concentrated our efforts on the pre-formulation
studies of the potential anticancer drug – Eu(III)
coordination complex (Fig. 1). Membrane-partitioning properties of the investigated drug were evaluated
using the equilibrium dialysis technique. To gain insight into the drug influence on
physical parameters and molecular organization of lipid bilayer, two fluorescent
probes have been employed, viz. 1,6-diphenyl-1,3,5-hexatriene
(DPH), and 4-p-(dimethylaminostyryl)-1-dodecylpyridinium (DSP-12). Liposomes were prepared from
zwitterionic lipid phosphatidylcholine (PC) and its mixtures with cholesterol (Chol) and cetyltrimethylammonium bromide (CTAB).
 Equilibrium
dialysis measurements. Equilibrium dialysis is a simple but effective
tool for the study of interactions between molecules. It can be employed to
measure separately the concentrations of bound and unbound ligand. In this
method, the actual concentrations of interacting species are measured after
equilibrium has been established between two compartments separated by a semipermeable membrane. Equilibrium dialysis is often used
as a reference method for the determination of liposome/water partition
coefficients. In terms of equilibrium dialysis methodology the
mole fraction partition coefficient is defined as:
Equilibrium
dialysis measurements. Equilibrium dialysis is a simple but effective
tool for the study of interactions between molecules. It can be employed to
measure separately the concentrations of bound and unbound ligand. In this
method, the actual concentrations of interacting species are measured after
equilibrium has been established between two compartments separated by a semipermeable membrane. Equilibrium dialysis is often used
as a reference method for the determination of liposome/water partition
coefficients. In terms of equilibrium dialysis methodology the
mole fraction partition coefficient is defined as:
![]() (1)
(1)
where ![]() and
and ![]() are the drug optical
densities at 266 nm in liposome-free and liposome-containing systems,
respectively. Partition coefficient determined in this way was found to be ca. 5×104.
The results presented here strongly suggest that the examined europium
coordination complex can be efficiently entrapped by the lipid phase of
liposomes.
are the drug optical
densities at 266 nm in liposome-free and liposome-containing systems,
respectively. Partition coefficient determined in this way was found to be ca. 5×104.
The results presented here strongly suggest that the examined europium
coordination complex can be efficiently entrapped by the lipid phase of
liposomes.
Effect of lanthanide on DPH anisotropy
The
drug-induced modification of hydrophobic membrane region was explored with
fluorescent probe DPH (1,6-diphenyl-1,3,5-hexatriene) whose steady-state
fluorescence anisotropy depends on molecular order of lipid acyl
chains. This parameter is frequently regarded as correlating with fluidity (or,
inversely, microviscosity) of a membrane, decreased
anisotropy mirrors the faster probe rotation. It was found that incorporation of investigated
compound into PC:CTAB liposomes is followed by the decrease of anisotropy value
(by 10-20.%, however DPH anisotropy was insensitive to drug incorporation into PC:Chol vesicles. The above effects can be explained in terms
of drug ability to modify molecular organization of a lipid bilayer.
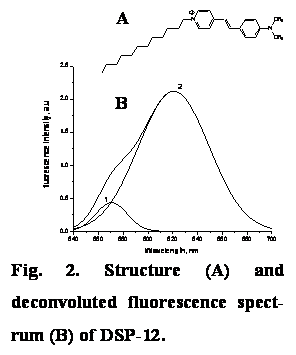 DSP-12 fluorescent measurements. DSP-12 (Fig.
2) is a fluorescent probe, which has a charged hydrophilic fluorophore moiety and a long hydrophobic tail. The alkyl
tail of DSP-12 tends to
locate in the hydrophobic membrane region, whereas aromatic group
resides at lipid-water interface. Drug-lipid binding was accompanied by the decrease of DSP-12 fluorescence
intensity. Analysis of DSP-12 emission spectra revealed two spectral components
with maxima 570 and 620 nm. However, contribution of the shorter- and the
longer-wavelength component remained unchanged upon incorporation of the
investigated compound into lipid bilayer. These findings suggest that the hydrophobic interactions play predominant
role in the membrane interactions of the Eu(III)
coordination complex. Insignificant bilayer-modifying effects in combination with appreciable lipophilicity are favorable for the development of
liposome-based carriers of this potential anticancer agent.
DSP-12 fluorescent measurements. DSP-12 (Fig.
2) is a fluorescent probe, which has a charged hydrophilic fluorophore moiety and a long hydrophobic tail. The alkyl
tail of DSP-12 tends to
locate in the hydrophobic membrane region, whereas aromatic group
resides at lipid-water interface. Drug-lipid binding was accompanied by the decrease of DSP-12 fluorescence
intensity. Analysis of DSP-12 emission spectra revealed two spectral components
with maxima 570 and 620 nm. However, contribution of the shorter- and the
longer-wavelength component remained unchanged upon incorporation of the
investigated compound into lipid bilayer. These findings suggest that the hydrophobic interactions play predominant
role in the membrane interactions of the Eu(III)
coordination complex. Insignificant bilayer-modifying effects in combination with appreciable lipophilicity are favorable for the development of
liposome-based carriers of this potential anticancer agent.
References
1.
2. Torchilin V. (2005) Recent advances with liposomes as pharmaceuticals carriers. Nature reviews. Drug
Discovery 4:145–160
3. Momekov G., Deligeorgiev
T., Vasilev A., Peneva K., Konstantinov S., Karaivanova M.
(2006) Evaluation of the cytotoxic and pro-apoptotic activities
of Eu(III) complexes with appended DNA intercalators in a panel of human malignant cell lines. Med
Chem 2:439–445