Berillo
D.A., Turmukhanova M.Zh., Abilov Zh.A., Ahmedova Sh. S.
Al-Faraby
Kazakh National University, Almaty, Kazakhstan
Synthesis of derivatives of† α-methyl-β-(N-piperidyl)-
propane acids
For a number of years we have conducted research work
in the basic directions, connected with updating heterocyclic amines, by introduction
of various the pharmacophore groups into their structure leading to the
decrease in toxicity of compounds, to strengthening of the basic
pharmacological effect and revealing of new kinds of biological
activity.
In the present
work for synthesis of potentially biologically active substances commercially
available reagents, such as methyl and butyl ester of methacrylic acids, piperidine,
hydroxylamine and 2,4-dinitrophenylhydrazine are used.
The presence of various functional groups in a piperidine cycle allows to
use piperidine as basic synton in organic synthesis and to consider its
derivatives as possible precursors of biologically active compounds. Availability
of several electrophilic centers with different reactivities assumes numerous
variants of interaction of such compounds with nucleophiles.
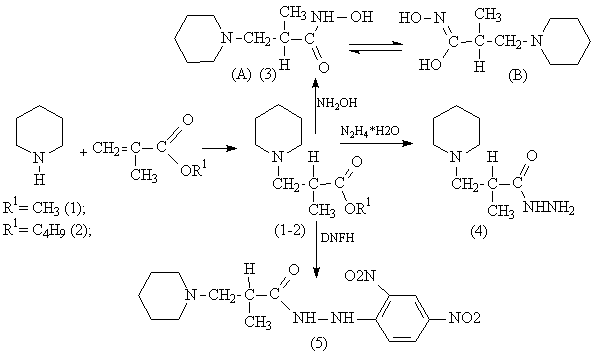
Synthesis of ester
of α-methyl-β-(N-piperidyl)propane acids (1,2) is
provided by nucleophilic addition of piperidine to methacrylic acid esters in alcohol
solution, at the same time there proceeds a reaction of reesterification.
It is known that hydroxamic
acids and metalorganic complexes of hydroxamic acid exhibit an inhibitor †activity on α-chymotrypsin and other enzymes [1,2]. With the purpose of searching
for potentially biologically active compounds α-methyl-β-(N-piperidyl)propane
hydroxamic acid is obtained as a result of reaction of† the methyl ester of α-methyl-β-(N-piperidyl)propane
acid (1) with hydroxylamine in the presence of alkali in the water medium. It is
well known that hydroxamic acids are characterized by keto-enol tautomerism [2]. So, in a crystal condition α-methyl-β-(N-piperidyl)propane
hydroxamic acid mainly exists in keto- form (A), that has
been proved on the basis of IR-spectroscopy by a characteristic absorption †band of a carbonyl group at 1649 см-1. As a consequence of spectrophotometric investigation
it is stated that in water medium the compound (1) represents a mixture of tautomeric forms A
and B.
Hydrazides of carbonic
acids are used as in quality hardeners for epoxy pitches and also are successfully
used in agriculture as herbicides, insecticides. Among hydrazine derivatives,
the effective medicinal substances being used in medicine are known. Among
antibacterial medicines there is a group of the antituberculosis means
containing a hydrazides fragment [3, 4].
One of the general
ways of synthesis of hydrazides is interaction of esters of carbonic acid with hydrazine
hydrate. Hydrazide of α-methyl-β-(N-piperidyl)propane acid was obtained
by condensation reaction of methyl or butyl esters of α-methyl-β-(N-piperidyl)propane
acid (1,2) with hydrazidehydrate in ethanol medium. A primary biological
screening for compound (4) has shown the following kinds of activity: spasmolitic,
analgesic and antimicrobic. It has been stated that compound (4) possesses low
toxicity (LD50 800 mg/kg) [5].
From literature
data it is known that derivatives of nitrocompounds show high antimicrobic
activity [3]. With the purpose of searching for an active medicine of a
wide spectrum of action, we carried out synthesis of N1-(2,4-dinitrophenyl)hydrazide of α-methyl-β-(N-piperidyl)propane acid (5) at condensation of 2,4-dinitrophenylhydrazine with a methyl ester
α-methyl-β-(N-piperidyl)propane acid (1) in
the presence of catalytic amounts of piperidine in the ethanol medium. Thus, functionalization
of the methyl ester of α-methyl-β-(N-piperidyl)propane acid (1) was
carried out in which resulted a combination of several pharmacophoric groups: the cycle of piperidine, hydrazide and
nitro Ц groups in compound (5). The composition and the structure of the obtained
compounds (1-5) were proved on the basis of data of IR- UV- NMR- spectroscopy
and GH-MS.
References
1.
A. Moulin, J. H. Bell, R.F.
Pratt, D. Ringe. Inhibition of Chymotrypsin
by a Complex of Ortho-Vanadate and Benzohydroxamic Acid: Structure of the Inert
Complex and its Mechanistic Interpretation. 2007, Biochemistry, Vol. 46 (20),
pp. 5982Ц5990.
2.
K. Kobashi, N.Terashima, J.
Hase. Spectrophotometric studies on hydroxamic acid-boronate and alumimate
complex. 10, 1973, Chem. Pharm. Bull., Vol. 23, pp. 2303-2308.
3.
M. D. Mashkovsky. Medical remedy. Kharkov: 1998. V.
1,2.
4.
є21703 The innovative patent R.K. D.A. Berillo, Sh.S. Ahmedova, S.N.
Shin, O.A. Berillo Hydrazide of 2-methyl-3-(N-piperidyl)propane
acid† possessing a spasmolitic,
analgetic and antimicrobial activity.†
(15.09.2009), bulletin є9.