THE EFFECT OF TITANIUM CHLORIDES ON CORROSION
RESISTANCE OF MATERIALS
Ivanov I. I.,
Dubovikov O.A., Kuzhaeva À.À.
Saint-Petersburg State Mining University
The corrosion stability of some pure metals and
alloys in the titanium(II) and (III) chloride melts was under investigation. It
was demonstrated that the rate and specific features of the process depend upon
the chemical composition of alloys. Under these conditions chrome-containing
alloys are most influenced to the corrosion. The relative corrosion stability
data of some materials were obtained.
The stability of steels and
metals in melts
containing titanium (II) and (III) chlorides, is of
considerable interest especially in
obtaining high purity titanium. It was found that the
corrosion rate of steel is mainly
determined by the composition of the medium [1]: the most active component is titanium(IV)
chloride , and then the titanium (III) chloride. The high content of titanium
in molten titanium (II) chloride
leads to its disproportionation
on the steel surface to form the metallic and intermetallic coatings. Comparison of different types of steels (St.-3,
1H18N9T, 2H13) shows
that Steel St.-3 in
the melts containing titanium (III) chloride is more stable than other
brands that exhibit selective dissolution of
chromium. Authors found
the decrease of corrosion resistance in a following series of the pure
metals: Ni, Mo, Cr, V, Mn. S.I. Stepanov
[2] noted that the corrosion of metals in
molten chlorides has
generally electrochemical nature, and
selective dissolution of certain metal alloys components can be explained by the difference of electrochemical potentials
of these elements. The effect of temperature and duration of the recovery process, as well as the content of titanium (III) chloride in the melt
on the corrosion of steel is reported by S.V.
Alexandrovsky [3]. Decrease in the relative content of titanium dichloride
enhances corrosion of constructional steels.
All the studies mentioned above were made
in static conditions, in which the diffusion processes are known to play a great role. Since the
melts are mixed intensively during metallothermic production and
purification from the impurities,
it seems necessary to clarify the stirring effect on the materials corrosion resistance.
Study of the materials corrosion in
the melt under agitation was carried out using the technique and the
installation described in [4]
at 800 º C
and the intensity of quartz ampoule shaking as much as 240 oscillations per minute with an exposure time 30 minutes.
It is evident (Table 1) that the most
aggressive component of the
considered melts is titanium (III) chloride: a melt of sodium
chloride and titanium
trichloride has a major corrosive
effect on all
the materials under investigation, except
for molybdenum and nickel.
Table
1
Summary of materials corrosion resistance research
|
Sample material |
The composition of the
samples used, |
Corrosion rate, |
|||
|
Russian translation |
English translation |
TiCl3 |
TiCl2 |
NaCl |
|
|
Ñò.3 |
St. 3 |
40 - - |
- - 50 |
60 100 50 |
110.6 2.1 0 |
|
1Õ18Í9Ò |
1H18N9T |
40 - - |
- 50 - |
60 50 100 |
164.2 0 1.3 |
|
ÝÈ-868 |
EI-868 |
40 - - |
- 50 - |
60 50 100 |
28.4 5.2 2.9 |
|
Ti |
Ti |
40 - |
- - |
60 100 |
30 8.8 |
|
Ni |
Ni |
40 |
- |
60 |
0.5 |
|
Mo |
Mo |
40 |
- |
60 |
0 |
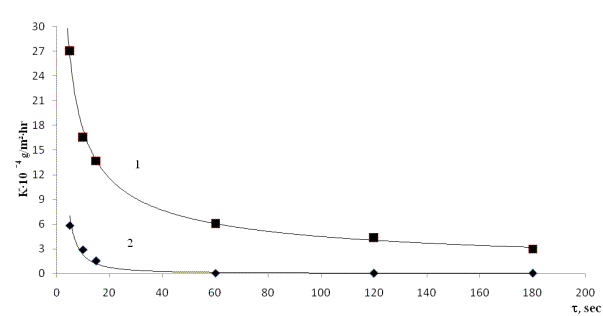
Fig. 1. The study
of corrosion St.-3 and VT
1 in the NaCl melts containing 12.3% Ti3+, and 0.40% Ti2+
1 – VT
1 (high purity compact titanium), 2 –
St-.3.
Despite
the significant difference in the rate of
destruction of titanium and
St.-3 (Fig. 1), the
corrosion of these materials in melts containing
titanium trichloride in the initial period of
interaction is characterized by the
same order of magnitude and then approaches to the
minimal positive value. It is evident from the following data that corrosional
destroying of 1H18N9T and EI-868 is caused to a
large extent by the selective
dissolution of chromium:
Table
2
|
Sample
material |
Composition of the salt
medium before the experiment, wt.% |
The content1 of impurities in the melt after the
experiment, wt.% |
||
|
TiCl3 |
NaCl |
Cr |
Ni |
|
|
1H18N9T |
40 |
60 |
1.06 |
ñëåäû |
|
EI-868 |
40 |
60 |
0.44 |
0,47[1] |
Only in the tests with EI-868 nickel which is known to be
the main component of this alloy was eventually found at the melt. It seems
that the transition of nickel into the melt in this case occurs in
conjunction with chromium due to the
non-crystalline corrosion.
The stainless steel corrosion study demonstrates the absence of nickel in the
melts after exposition; the higher corrosion resistance can be caused by the
cementation by chromium at the inox steel.
An additional data on the
constructional materials (St.-3, 1H18N9T, EI-868)
corrosion in melts containing titanium
trichloride were obtained
by the use of the rotating disk with equally
accessible surface [4, 5].
The metal disk immersed in melt was
rotated at a given rate within a
certain period of time, after which the
melt was raised,
cooled, washed out of chloride salts, dried and
weighted.
Dissolution rate was
determined by weight loss:
![]()
where: - n - the
dissolution rate, mg / cm2 •min;
Δm - weight loss,
mg;
![]() - the
surface of the sample contacting with the melt, cm2;
- the
surface of the sample contacting with the melt, cm2;
![]() - time of exposure,
min.
- time of exposure,
min.
Duration of contact material with the melt was
chosen empirically, for the
obtaining a sufficient weight loss as
a criteria, and to be possible ignore the amount of dissolved metal, corresponding
to the conditions of the
rotor method [4]. For all materials
under investigation it was established the
exposure time can be varied at the range
of 1-10 min for the melt containing 4 wt.% Ti.
When the disc
rotation speed changes from 240 to 830 rpm, the
linear dependence of dissolution rate of
titanium on the square root of rotation
speed occurs (Fig.
2), which seems to be a consequence of laminar flow over the disc.
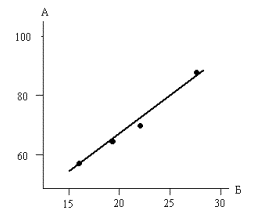
Fig.2 Dependence
of the dissolution rate of
titanium on the angular
velocity of the sample.
A - dissolution
rate, mg / cm2• min
B - angular
velocity of the sample ![]() min
min![]()
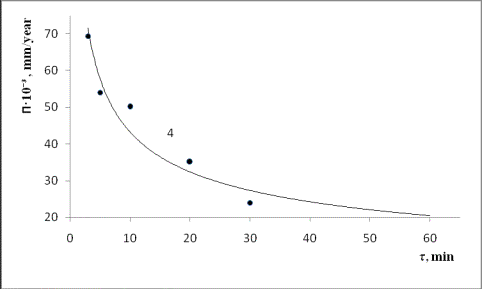
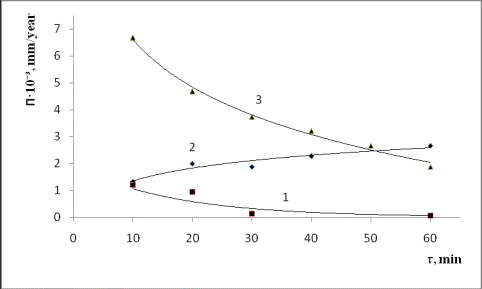
Fig.3.
The kinetics of corrosion of structural materials
1– EI-868; 2 – 1H18N9T; 3 – ST.-3;
4– VT-I.
The summary data,
which allow to characterize the materials
corrosion resistance are
shown at Fig.3. For comparison,
curve 1 at this figure presents the destruction
intensity of high purity compact titanium
(VT-I) samples.
The destruction rates of the specimens VT-I, St.-3, EI-868 are
reduced with increasing exposure
time which is probably caused by disk surface
passivation due to the accumulation of. low-reactivity
substances thereon. These
inactive surface substances are expected to form oxide and nitride films. The
behavior of stainless steel
pattern with respect to other materials is
unusual; the destruction rate of the inox
steel at the first time increases
and afterwards
demonstrates the trend to stabilize at the
definite rather high level. This phenomenon may be caused by high-rate interactions of the
melt with chrome, one of the main components
of the stainless steel.
Thus, we conclude
that the most durable construction
material in the melts enriched with
titanium trichloride, is EI-868.
Due to its high thermal
stability, this material can be used for the
manufacturing of the multipurpose
valves. The St.-3 and EI-868 are
characterized by close values of the corrosion
resistance at a significant exposure
time. Destruction rate of titanium is 5-10 times higher
than that of all other materials
under the investigation.
The results of these studies are
well-correlated with the data
obtained by the study of the interaction of metal
samples with melts in a sealed,
intensive-shaking quartz cells.
One of the main results of the present study is that the usage of high-chromium steels which are known to have high
chemical stability towards the major part of corrosion and surface oxydation
processes is strictly undesirable in manufacturing the equipment for the melts enriched with
titanium trichloride.
Literature
1.
V.G. Gopienko, G.N. Gopienko, V.V. Timofeev,
and D.I. Podushkin /Behavior of steels in melts containing titanium
chlorides. // Journal of Applied Chemistry. 1966. V. 39,
No.6. P.1249.
2.
S.I. Stepanov. Physical chemistry of molten salts.
Moscow: Metallurgiya,
1965. P. 340.
3.
S. Alexandrovsky, Ph.D. Thesis. Leningrad, 1966. 15 p.
4.
R. Sandler et al. / Izv. Vuzov
(Russian-Soviet edition: "News of the High Schools"). "Non-ferrous
metallurgy", 1976. ¹ 5. P. 31-35.
5.
V.G. Levich. Physico-chemical
thermodynamics. Princeton, 1959.
699 p.