ÓÄÊ 546.212:
541.12.012.3+534-14
OPTICAL AND ACOUSTIC EMSSION
MTHODS USING FOR INVESTIGATE OF THE WATER STRUCTURES
A.N. Smirnov
Moscow State Technical University Radiotechnics, Electronisc and
Automatics,
78 Vernadsky prosp., Moscow,
119454, Russia E-mail: a.n.smirnov@mail.ru
In
previous studies [1] it was shown that liquid water has a very complex
structure. Using optical methods,
acoustic emission and by thermal analysis of the water supramolecular
complexes sized from 1 to 100 μm (micrometre) were
found in “continuous“ aqueous systems. Basing on the characteristic properties
of these supramolecular formations we have named them “emulons”. Sizes and
spatial organization of supramolecular complexes depend on the composition of
aqueous solutions, temperature and prehistory of the water. Size
specters of emulons reveal five fractions with characteristic sizes:1-3 μm,10-12 μm,30-35 μm, 70 μm è100 μm. As it can be seen organization and distribution of
supramolecular complexes - emulons, their appearance in “continuous” water depends on chemical
composition of aqueous solution and thetemperature.
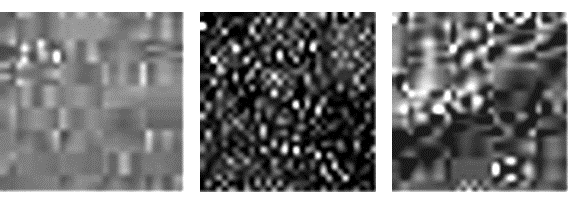

Fig.1.
Influence of the temperature on the structure of water
Effect of the temperature on the water structure 
 is presented on figure 1. At 4°Ñ
emulons are densely packed and form a texture similar to parquet. As we know,
water at this temperature has maximum density. After increasing of water
temperature to 20°Ñ its structure is drastically changing – number of free
emulons reaches it maximum. After that during further increase of temperature
they gradually dissipate, their number decreases and this process usually ends
at 75-80°Ñ. This fact explains many abnormalities of water, also the highest
speed of sound in water at 75°Ñ.
is presented on figure 1. At 4°Ñ
emulons are densely packed and form a texture similar to parquet. As we know,
water at this temperature has maximum density. After increasing of water
temperature to 20°Ñ its structure is drastically changing – number of free
emulons reaches it maximum. After that during further increase of temperature
they gradually dissipate, their number decreases and this process usually ends
at 75-80°Ñ. This fact explains many abnormalities of water, also the highest
speed of sound in water at 75°Ñ.
This means, that acoustic emission method (AE) is a very powerful
experimental method for investigate of the
water structurs, during any kind of chemical reactions and
physical-chemical processes. In our experiments we have used modern acoustic
emission system ALine32. Acoustic
emission during dissolution process of different salts in the water show in the
taible
Acoustic emission
procesing dissolution of different
salts in the water (1N solutions).
|
Parameter |
Li2SO4 |
KCl |
|
Overall count of AE impulse imp. |
3320 |
1100 |
|
AE activity,
imp./sec |
96 |
32 |
|
Amplitude, dB |
33-41 |
38 |
|
Energy, dB |
62-80 |
63-75 |
|
Duration, µsec |
400 |
100-1000 |
|
Increase time of amplitude, µsec |
~50 |
600 |
|
Number of oscillations, imp. |
30 |
20 |
As we can see from the
table , number of impulses, AE activity, duration and time of signal amplitude
increase are very different for dissolution of various salts metals in the water.
Complex organization of water structure as a unite ensemble, that
includes supramolecular complexes - emulons, result in the fact that properties
of aqueous system are not simply the sum of properties of its different
structural elements, but are explained by cooperation phenomenon . The
polydisperse structure of the emulons formed
of the water, ensuring polymodalnost reply by the external affects, appearance hysteresis, considderable
times relaxation. Since the water,
in at many cases is a primary target
for faint exercise influence on the biology systems, it is posssible the
structure of water modification in the
time investigation pay attention very much.
Summary
OPTICAL AND ACOUSTIC EMSSION
MTHODS USING FOR INVESTIGATE OF THE WATER STRUCTURES
Smirnov
A.N., Moscow State Technical University Radiotechnics, Electronisc and
Automatics, 78 Vernadsky prosp., Moscow,
119454, Russia E-mail: a.n.smirnov@mail.ru
The
using optical method and acoustic
emission method for the water structure analysis are suggested. Sizes and spatial organization of emulons
depend on the composition of aqueous solutions, temperature and prehistory of
the water. The polydisperse structure of the water, ensuring polymodalnost
reply by the external affecns.
References
Smirnov A.N. Water structure: new
experimental date. // Science and Technologies for the industry, 2010, ¹ 4,
pp. 41…45.
Key words: optical methods, acoustic emission, water structure, salt dissolution kinetics.