color, texture and lipid oxidation of
comminuted meat products with oat and sodium isoascorbate addition
Z.J. Dolatowski, M. Karwowska
Department of Meat Technology and Food Quality, Agricultural University of Lublin,
8 Skromna St,
20 - 704 Lublin, Poland
Key Words: Meat product, color, lipid
oxidation, oat, sodium isoascorbate
Introduction
Oxidation processes in food systems
deteriorate the sensory quality and nutritive value of a product; these
processes frequently limits the shelf-life of meat products. Lipid and protein oxidation
is responsible for the development of unpleasant tastes and odors, as well as
changes in reological properties and formation of
toxic compounds (Kanner, 1994). Moreover it
deteriorates the color of meat products. The studies concerning meat haem pigments initiate and catalyse
the oxidation of muscle tissue. Free radical, produced during lipid oxidation,
can oxidise haem pigments,
causing discolouration of meat and meat products. Myoglobin catalyses lipid oxidation; iron ions (Fe2+,
Fe3+) present in haem are better prooxidants than as free ions (Baron & Andersen, 2002). One method to reduce oxidation processes
is the application of natural antioxidants. Oat grains are a basic source of
essential components with a wide range of biological activity, e.g. polyphenols. Oat phenolics include
simple phenolics, such as ferulic
acid, caffeic acid, p-coumaric acid and vanillin, in free and bound
forms, and flavonoids such as kaempferol
and quercetin (Xing and White, 1997). The avenanthramides
belong to a group of phenolic compounds which
are unique to oat (Dimberg et al., 1993).
The
aim of the study was to investigate the effects of oat grains and sodium isoascorbate addition on the oxidative stability of
comminuted meat products.
Materials and Methods
Experimental
material consisted of finely comminuted meat products. Fundamental materials
used for manufacturing the test products
were: cured lean beef – 25%, cured pork meat – 25%, minced pork fat – 20%, ice
water – 30% and oat grains (2 and 5%) and sodium isoascorbate
(0,05%). Oat grains (Polar) were purchased from a local grain manufacturer in
Color measurement. Hunter color lightness (L*), redness (a*) and yellowness (b*) values
were measured on freshly cut surfaces of each sample using X-Rite reflection spectro-colorimeter, using illuminant D65 and 10° observer
angle (AMSA 2005). ΔE* (total color change) values were calculated (![]() =
=![]() ) to determine the extent of color change.
) to determine the extent of color change.
Acid number values (AV) were measured in accordance with PN- 84/A – 85803 and expressed in
milligrams of KOH/
Lipid oxidation was assessed by the
2-thiobarbituric acid method. The rose-pink colour obtained through the
reaction between malondialdehyde and 2-thiobarbituric
acid was measured at 532 nm using a Nicole Evolution 300 spectrophotometer
(Thermo Elektron Corporation). The TBA content was
expressed as mg of malondialdehyde per kg of the
samples.
Instrumental evaluation of the texture. Instrumental
texture profile analysis (TPA) was used to evaluate the instrumental texture
using a texturometer TA XTplus
(Stable Micro Systems,
Statistical analysis. To compare mean values of the investigated parameters, analysis of
variance was applied and differences between groups were evaluated using Tukey test.
Results and Discussion
Color and color stability. There were no significant differences (P>0,05)
between all experimental meat products during storage for lightness (L* value)
and yellowness (b* value). L* and b* values of control sample (PC) tended to be
slightly higher (but not significantly) when compared with the products with
sodium isoascorbate (PSA) and oat (PO1, PO2)
addition. Statistical analysis indicated that the addition of sodium isoascorbate and oat preparation did affect the redness (a*
value). The control sample (PC) characterized significantly lower a* parameter
values compared to the rest of meat products samples. During the chilling
storage of meat products slight changes in a* parameters were noted. The total
color change (ΔE*) of control meat products was significantly (P>0,05) higher compared to the products
with sodium isoascorbate and oat addition (Table 1).
Some authors linked color changes of cooked meat products during storage with
lipid oxidation (Akamittath et al. 1990; Jo et al.
1999); it is reasonable since the addition of substances with antioxidant
activity inhibit to same extent discolouration of
meat products.
Table 1. Hunter colorimetry
and color stability of meat products stored at
|
Sample |
Color
parameter |
ΔE* |
|||
|
L* |
a* |
b* |
|||
|
PC |
1 day |
70,26a |
4,58a |
13,90a |
- |
|
15 days |
69,01a |
5,98a |
13,14a |
2,03a |
|
|
30 days |
70,40a |
6,60b |
11,21a |
3,37a |
|
|
PSA |
1 day |
69,16a |
11,09c |
11,43a |
- |
|
15 days |
68,78a |
10,87c |
11,82a |
0,58b |
|
|
30 days |
69,24a |
10,92c |
11,48a |
0,20d |
|
|
PO1 |
1 day |
69,15a |
10,74c |
11,11a |
- |
|
15 days |
68,89a |
11,23c |
11,97a |
1,02c |
|
|
30 days |
69,14a |
10,62c |
11,84a |
0,73c |
|
|
PO2 |
1 day |
68,89a |
10,48c |
12,23a |
- |
|
15 days |
68,51a |
10,71c |
12,32a |
0,46b |
|
|
30 days |
69,16a |
10,45c |
11,85a |
0,47b |
|
Averages marked with the same letters are not significantly
different (P>0,05)
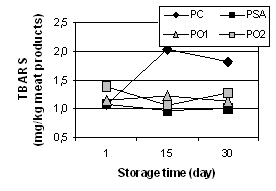
Lipid oxidation. The addition of sodium isoascorbate
and oat preparation had an effect on TBARS values (Figure 1). After 15 and 30
days since the production, the rate of TBARS values was higher for control
sample than for the samples with the sodium isoascorbate
and oat preparation addition. Slight differences in TBARS values for PSA, PO1
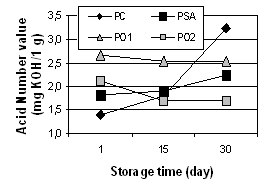
and PO2 samples during 30 days of storage were noted. The acid number
evaluation showed that the control characterized the highest acid number value
after 30 days since the production.
|
|
|
Figure 1. Lipid oxidation of meat products
stored at
Texture profile analysis. Results from the texture
profile analysis of comminuted meat products during 30 days of storage are show
in Table 2. All texture parameters were affected by the addition of oat grains
and sodium isoascorbate except the cohesiveness and
elasticity. The addition of oat grains in model comminuted meat products
affected the changes of hardness I and II, gumminess and chewiness. Significant hardness increase was
recorded for products with 2 and 5% oat grains addition. During 30 days of
chilling storage the texture parameters did not change significantly in almost
all group of meat products. The influence of oat grains on a texture may be
elucidated by the high ability of water and fat retention as well as formation
of a strong gel. According to Gray (1978) lipid oxidation affects essential
sensory traits of meat products texture deterioration. Protein oxidation is also
believed to affect protein functionality and their emulsification ability (Xiong, 2000); it is possible that protein oxidation caused
texture deterioration through the loss of protein functionality.
Table
2. Texture parameters of meat products stored at 4°C
|
Sample |
Texture parameters |
||||||
|
H I [N] |
H II [N] |
C |
E [mm] |
G [N·mm] |
CH [N] |
||
|
PC |
1 day |
17,15a |
13,71a |
0,62a |
0,83a |
10,90ab |
9,09ab |
|
15 days |
19,70ab |
15,21a |
0,58a |
0,79a |
10,95ab |
9,49ab |
|
|
30 days |
17,11a |
12,99a |
0,49a |
0,80a |
8,42a |
7,72b |
|
|
PSA |
1 day |
17,15a |
13,71a |
0,62a |
0,83a |
10,90ab |
9,09ab |
|
15 days |
20,01ab |
16,45b |
0,64a |
0,85a |
11,92ab |
11,01a |
|
|
30 days |
22,14b |
16,42b |
0,59a |
0,85a |
13,07b |
11,09a |
|
|
PO1 |
1 day |
23,39b |
20,03c |
0,67a |
0,82a |
16,10c |
13,15ad |
|
15 days |
22,74b |
15,53b |
0,55a |
0,80a |
12,57b |
10,05a |
|
|
30 days |
21,17b |
17,39b |
0,65a |
0,86a |
13,92b |
11,95a |
|
|
PO2 |
1 day |
30,71c |
20,95c |
0,55a |
0,81a |
16,90c |
13,77ad |
|
15 days |
29,52c |
24,77c |
0,65a |
0,81a |
19,07c |
15,22d |
|
|
30 days |
27,37c |
17,40b |
0,51a |
0,85a |
14,08b |
12,02a |
|
Averages marked with the same letters are not
significantly different (P>0,05)
Conclusions
Color and texture of meat products
are important quality attributes that influences consumers’ acceptance. The
addition of oat and sodium isoascorbate affected the
changes of oxidation stability of comminuted meat products. The result
indicated that oat grains and sodium isoascorbate
reduced lipid oxidation, and maintained redness (a* value) and prevented texture
deterioration during 30 days of chilling storage. It is suggested that natural antioxidants
which are present in oat grains prevented myoglobin
formation and lipid oxidation.
References
1.
Akamittath J.G., Brekke C.J., Schanus E.G. 1990.
Lipid oxidation and color stability in restructured meat systems during frozen
storage. Journal of Food Science, 63, 15-19.
2.
American Meat Science Association (AMSA) 2005 –
Guidelines for Meat Colour Evaluation – AMSA,
3. Baron C.P., Andersen H.J. 2002. Myoglobin – induced lipid oxidation. Journal Agriculture
Food Chemistry, 50, 3887-3897.
4. Bilska A. 2006. The effect of the addition
of isoascorbic acid and sodium ascorbate
on sensory quality of raw sausage. Acta Scientiarum Polonorum. Technologia Alimentaria, 5(1),
143-154.
5. Bourne M.C. 1978. Texture Profile Analysis.
Food Technology, 32, 62-66.
6. Dolatowski Z.J.,
Karwowska M. 2006. An
assessment of fat substitution with buckwheat seed preparation on the quality
of comminuted meat products.
7. Dimberg L.H., Theander
O., Lingnert H. 1993. Avenanthramides - A group of phenolic
antioxidants in oats. Cereal Chemistry, 70, 637–641.
8. Folch, J., Lees, M., & Stanley, S.G.H.
9. Gray J.I. 1978. Measurement of lipid
oxidation: a review. Journal American Oil Chemistry Sociation,
55, 539-546.
10. Jo C., Lee J.I., Ahn D.U. 1999. Lipid oxidation, color changes and
volatile production in irradiated pork sausage with different fat content and
packaging during storage. Meat Science, 51, 355-361.
11. Kanner J. 1994. Oxidative processes in
meat and meat products: quality implications. Meat Science, 36, 169–189.
12. Xing Y., and White P.J. 1997.
Identification and function of antioxidants from oat groats
and hulls. Journal American Oil Chemistry Sociation, 74, 303–307.
13. Xiong Y.L. 2000. Protein oxidation and
implications for muscle foods quality. In: Decker E.A., Faustman
C., Lopez-Bote C.J., editors. Antioxidants in muscle
foods.