I.V. Khritankova,
O.A. Lytkina, M.S. Kukharskiy
Institute of Physiologically Active Substances,
Russian Academy of Sciences,
1 Severnyi proezd, Chernogolovka, 142432, Moscow
Region, Russian
Dimebon
stimulates activation of autophagy-related events in cell culture
INTRODUCTION
Dimebon was originally designed as an antihistamine drug but it
experienced a revival of interest in connection with neurodegenerative
disorders [1]. Dimebon inhibits aggregation of aggregate-prone proteins in cell
cultures [2] and slows proteinopathy progression by hindering amyloid inclusion
formation in neuroblastoma cells of transgenic mice [3, 4]. Nutrient depletion
as well as other environmental cues such as immune defense can induce autophagy. The complexity of autophagy regulation suggests that signal timing and
intensity are important in conveying the right physiological message. ERK1/2
pathway inhibition by pretreatment with PD98059 suppresses autophagy [5]
whereas sustained ERK activation for more than 24 hours can block autophagosome
maturation [6]. Failure to remove
protein inclusions often cause neurodegeneration and this was
shown on a few animal models such as conditional deletion of Atg5 (autophagy-related 5) in mouse neurons. Atg5 conjugates with Atg12 and
subsequent addition of Atg16 forms an ubiquitin-like conjugating system
recruited to autophagosome membrane to mediate its expansion [7]. Concomitant
expression shifts in all three members of the complex might serve as an
indication of actived autophagy program, therefore we decided to assess dimebon
effects on Atg12 and Atg16 in SH-SY5Y cells as well. Another objective was to
examine whether dimebon treatment affects MAP kinases activation, particularly
ERK1/2 and p38. ERK1/2 phosphorylation is commonly used as indicator of
proliferative or anti-apoptotic signalling and can induce autophagy in response
to anti-tumour agents. At the same time p38 is an important mediator of stress
response but its implication in autophagy has been controversial. Recent data
suggested that although p38 and ERK1/2 can be activated simultaneously, p38 can
inhibit ERK1/2 – directed autophagy [6]. The aim of this study was to assess whether dimebon has an impact on
autophagy-related gene transcription in SH-SY5Y human
neuroblastoma cell line.
MATERIALS
AND METHODS
SH-SY5Y human neuroblastoma cells were
cultivated in
DMEM/F12 medium (Sigma) supplemented with 10% fetal calf serum,
penicillin-streptomycin (10000 1U/ml-10000µg/ml),
L-glutamine (Sigma), and MEM non-essential amino acids solution (Gibco).
24 hrs after seeding the culture medium was supplemented with 10 μM of
dimebon and further incubated for 30 min, 1 hr, 2 hr, 3 hr
and 6 hrs. Cells were washed on ice with cold PBS and collected either for
protein extraction and western blotting or for RNA isolation, cDNA synthesis
and quantitative RT-PCR.
Protein
concentrations were determined using a Coomassie based method (Bio-Rad, USA).
Equal amounts of total protein (20 μg) were
separated on 10% polyacrylamide gel and subsequently transferred on Hybond-P
membrane (GE Healthcare, UK) for western blotting. HRP-conjugated secondary antibody
(Amersham Biosciences, USA) was used (dilution 1:3000) for protein detection
using ECL reagent (Amersham Biosciences, USA). The primary antibodies were as
follows: rabbit polyclonal phospho-p38 MAPK
(Thr180/Tyr182), 1:1000 (Cell Signaling, USA), phospho-p44/42
MAPK (Erk1/2) (Thr202/Tyr204), 1:1000
(Cell Signaling, USA) and rabbit polyclonal GAPDH, 1:1000 (Santa Cruz,
USA).
Gene
expression levels were analyzed via real-time, quantitative PCR. Total RNA was extracted using TRIzol (Invitrogen, USA)
and standard phenol-chloroform protocol. Reverse transcription was
performed with SuperScript III reverse transcriptase (Invitrogen,
USA) and random hexamers (Invitrogen, USA) according to the manufacturer’s instructions. The
PTC-200 Peltier thermal cycler (MJ Research) and Chromo4
fluorescence detector (MJ
Research)
were used in conjunction with Opticon Monitor analysis software (version 2.03, MJ Research) to
calibrate and run the reaction. The sequences for primers used were as follows (primers
were designed using primer3TM software):
human ATG5
CTCCGCGCCGGTGCTTTTTG (forward) and
CAGATTCCGCGCTCCGGTGG
(reverse),
human ATG12
CCCCGTCTTCCGCTGCAGTT (forward) and
TCGTGTTCGCTCTACTGCCCACT
(reverse),
human ATG16
AGCCCGGCTGCAGAAAGAGC (forward) and
TGCTCTGCTGATGGCTCGCA
(reverse),
human ribosomal
protein L13a GCATCCCACCGCCCTACGAC (forward) and
CCAGCCAACCTCGTGAGCCA
(reverse).
The
fold change was determined using 2-ΔΔCT method using ribosomal
protein L13a as a reference gene for all the samples.
RESULTS
Our data shows that dimebon treatment resulted in a
quick increase in ERK1/2 and p38 phosphorylation. Their activation levels
reached maximum within 30 minutes after the start of dimebon treatment but the signal lower
at 2hr and 6hr timepoints. Although
phospho-ERK signal intensity declined after the initial peak it was still
stronger than basal levels (Fig. 1, A). Transient ERK phosphorylation is
essential for autophagy whereas sustained and strong activation can block
autophagosome maturation [6]. Phospho-p38 levels followed a similar pattern in
dimebon-treated cells but it returned back to pre-treatment levels at 1hr
timepoint and decreased below the basal level at 2hr and 6hr (Fig 1, A). The data suggests
that dimebon not only stimulates a short-lived boost in ERK1/2 and p38
phosphorylation in SH-SY5Y cells but it can also elicit a more prolonged effect judging
from the ratio between phospho-ERK1/2 and phospho-p38 at the later timepoints. ERK1/2 and p38 are
known to be engaged in a complex interplay when regulating autophagy: ERK stimulation
activates autophagy, the concomitant p38 upregulation blocks
autophagic vacuolation. Strong and sustained p38 activation often corresponds
to stress-induced apoptosis whereas autophagy regarded as a survival mechanism.
ERK1/2
phosphorylation boost in dimebon treated SH-SY5Y cells was accompanied by
transcriptional increases in autophagy markers such as Atg5-Atg12-Atg16 ubiquitin-like
conjugating system (Fig 1, B). Real-time quantitative PCR data showed enhanced mRNA
levels for all three members of the complex: levels of Atg5 and Atg12
experienced a transient but smooth rise peaking around 2-3 hrs post-treatment
whereas Atg16 expression was maximal at 1 hr (Fig 1, B). Atg5 is
known to be covalently linked with Atg12 with Atg16 subsequently joining Atg12-Atg5 conjugate
[7].
The tight link between Atg5, Atg12 and Atg16 must be reflected
on a transcriptional level as well and the observed synchronized increase in
Atg5, Atg12 and Atg16 levels might indicate that
dimebon-treated SH-SY5Y cells experience an activation of autophagy program.
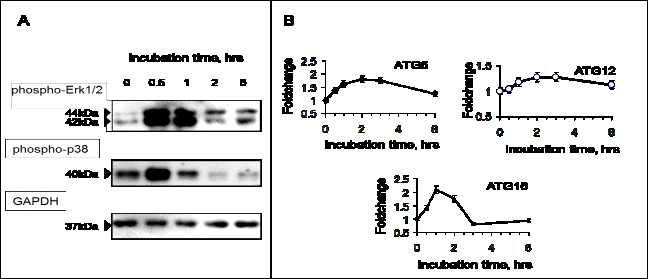

Figure
1 – Dimebon activates MAP kinases and induces autophagy-related gene
expression. A: western blot analysis of phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) and phospho-p38 MAPK
(Thr180/Tyr182) in SH-SY5Y cells. B: real time
quantitative PCR results showing increases in mRNA for ATG5-ATG12-ATG16 complex
CONCLUSION
Our
data shows that dimebon might stimulate autophagy response. We observed a
transient boost in ERK1/2 and p38 phosphorylation in dimebon-treated SH-SY5Y
neuroblastoma cells and this corresponded to activated transcription of several
autophagy-related genes: Atg5-Atg12-Atg16 ubiquitin-like conjugating system.
REFERENCES
1.
Doody S., Gavrilova S.I., Sano M., Thomas R.G., Aisen P.S., Bachurin
S.O., Seely L. and Hung D. Effect of dimebon on cognition, activities of daily
living, behaviour, and global function in patients with mild-to-moderate
Alzheimer’s disease: a randomised, double-blind, placebo-controlled study//
Lancet. – 2008 – vol. 372. – P. 207–215.
2.
Yamashita M., Nonaka T., Arai T., Kametani F., Buchman
V.L., Ninkina N., Bachurin S.O., Akiyama H., Goedert M. and Hasegawa M.
Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models//
FEBS Lett – 2009 – vol. 583. – P.2419–2424.
3.
Bachurin S.O., Shelkovnikova T.A., Ustyugov A.A., Peters O., Khritankova I., Afanasieva M.A., Tarasova T.V., Alentov I.I., Buchman V.L. and Ninkina N.N. Dimebon Slows Progression of
Proteinopathy in γ-Synuclein Transgenic Mice// Neurotox Res – 2011
– Epub 17 Dec 2011; PMID:22179976
4.
Bachurin S.O., Ustyugov A.A., Peters O., Shelkovnikova
T.A., Buchman V.L. and Ninkina N.N. Hindering of
proteinopathy-induced neurodegeneration as a new mechanism of action for
neuroprotectors and cognition enhancing compounds// Doklady Biochemistry and Biophysics – 2009 – vol. 428.
– P. 235-238
5.
Ogier-Denis E., Pattingre S., El Benna J. and Codogno P. Erk1/2-dependent
phosphorylation of Galpha-interacting protein stimulates its GTPase
accelerating activity and autophagy in human colon cancer cells// J Biol Chem. –
2000 – vol. 275(50). – P. 39090-5.
6.
Corcelle E., Nebout M., Bekri S., Gauthier N., Hofman P., Poujeol P., Fénichel
P., and Mograbi B. Disruption of autophagy at the maturation step by the
carcinogen lindane is associated with the sustained mitogen-activated protein
kinase/extracellular signal-regulated kinase activity// Cancer Res. – 2006 –
vol. 66(13). – P. 6861-70.
7.
Matsushita M., Suzuki N.N., Obara K., Fujioka Y., Ohsumi Y. and Inagaki
F. Structure of Atg5.Atg16, a complex essential for autophagy// J Biol Chem. –
2007 – vol. 282(9). – P. 6763-72.