Artur Mantel1, Nikolay
Barashkov2, Irina Irgibayeva1, Anton Kiriy3
and Volodymyr Senkovskyy3
1L.N.
Gumilyov Eurasian National University, Astana, Kazakhstan
2Micro
Tracers, Inc., San Francisco, California, USA
3Leibniz
Institute of Polymer Research, Dresden, Germany
Free-radical quaternary
copolymerisation of styrene, 4-vinylbenzoic acid, 9-vinylanthracene and
2-vinylnaphthalene: composition of prepared copolymers
Introduction
This study is devoted to the synthesis and
investigation of styrene and its carboxy-derivative copolymers containing
naphthalene and anthracene chromophore fragments into polymer chain. Copolymers
of styrene, including chromophore-containing copolymers has been previously
used as materials for efficient plastic scintillators1-4. Analysis
of previously reported data on copolymerization of styrene (Sty) with
comonomers, such as 9-vinylanthracene (9VA)
and 2-vinylnaphthalene (2VN) shows that 2VN and Sty possess close constants of
copolymerization5,6 and therefore all 2VN participates in reaction.
In contrary, 9-VA has been found to be less reactive than Sty and its
significant part (at least 50% according to data7) does not
participate in reaction of copolymerization. There are some experimental
evidences that besides reaction of copolymerization, 9VA is capable of
interacting with styrene in the following ways 3,8: a) addition of
styryl or polystyryl radicals to the
meso-position of the anthracene ring; b) addition of one of several molecules
of styrene to the vinyl group of 9VA to form low molecular weight products;
substitution of hydrogen atoms in the meso-position of the anthracene ring by
styryl or polystyryl radicals.
There are also
numerous papers describing the synthesis and application of carboxyl-containing
styrene-based copolymers. Carboxyl group is often used for the subsequent
modification9, which explains our intention to introduce the carboxy
group into the chromophore-containing polymer. Carboxylic group is introduced
into copolymer by the means of copolymerization of styrene with 4-vinylbenzoic
acid10 (VBA). Taking into account the available literature data, we
made an attempt to synthesize the quaternary copolymer, containing the predicted
ratio of the four monomers in the chain.
Experimental
Materials. All reagents were purchased
from the Aldrich Chemical Co. 2-vinylnaphthalene(2VN), 9-vinylnaphthalene (9VA)
and styrene (Sty) were purified from stabilizator, products of oligomerization
by silica column with hexane as eluent;
4-vinylbenzoic acid (VBA) was recristallized from water: ethanol (3:7 v/v);
2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidine was prepared from TEMPO
and (1-bromoethyl)benzene by the method presented below.
Instrumentation. Gel
permeation chromatography (GPC) was used to determine the molecular weights and
molecular weight distributions, Mw/Mn, of polymer samples with respect to
polystyrene standards. The system configuration: THF with flow rate 1.0 ml/min.
HPLC-Pump, Ser. 1200, Agilent Technology; ETA-2020 – RI – and
viscosity detector from Fa.Bures; MALLS detector from Wyatt.
1H NMR spectra
were collected on the device «Bruker Bio Spin» (1H 500 MHz, 20 0Ñ, solvent
-CDCl3, TMS - an internal reference).
UV/vis absorption
spectra were measured on Perkin Elmer Lambda 800 spectrophotometer.
Synthesis of
2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperi-dine. To the round-bottomed flask containing 0.5008 g of copper
bromide, which is closed with a septum and filled with argon , the mixture of 0.73
ml N,N,N',N'',N''-pentamethyldiethylenetriamine and 10 ml dry toluene was added.
To another flask equipped with a magnetic stirrer a mixture of 0.538 g (1-bromoethyl)benzene
and 0.5 g ÒÅÌÐÎ was added. The air was evacuated from the reaction mixture, 10
ml of dry toluene was added and a reaction flask was flushed with dry argon.
The content of first flask was added slowly to the content of second flask and
mixture was stirred for 1 hr at temperature 50 0Ñ.
The obtained
solution was purified by chromatography using toluene as eluent. After evaporation of toluene, the
oily liquid was slowly crystallized in the cold. The resulting white crystals were
dried in vacuum and characterized by 1H
NMR.
Copolymerization of styrene 2-vinylnaphtalene, 9-vinyl-anthracene
and 4-vinylbenzoic acid.
Mixture of VBA (5.5 mmol, 0.815g), TEMPO (0.031 mmol, 0.00478g) and 2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidine
(0.304 mmol, 0.07940g) was dissolved in 8 ml (69.59 mmol, 7.248g) of Sty. Mixture
was divided into two equal parts. One of them was placed in a flask containing
9VA (0.038 mmol, 0.00784g) and 2VN (0.142 mmol, 0.02189g). On next step both
parts were subjected to three freeze
thaw cycles to remove oxygen, and flasks were flushed with dry nitrogen. Both
mixtures were stirred overnight at the temperature 130 0C. Prepared
copolymers were dissolved in the system CH2Cl2:CH3OH 9:1 and precipitated in
hexane three times. Polymers are drying to constant weight in vacuum at
temperature 70 0C and characterized by 1H NMR.
Results and Discussion
Figure 1 shows the polymerization process and chemical structure of prepared
quaternary copolymer (CP). In case when l=n=0,
the corresponding copolymer without chromophore fragments (MX) has been
prepared.
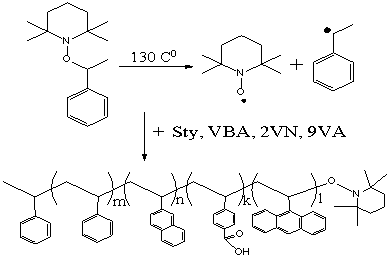
Figure 1. Copolymerisation of styrene
2-vinylnaphtalene, 9-vinylanthracene and 4-vinylbenzoic acid.
According to
GPC data, the Mw of copolymers CP and MX are
30,000 and 30,400, respectively. The determined molecular weight
distributions have been equal to 1.09 and 1.16 for CP and MX, accordingly. The
amount of VBA in copolymers has been determined by the titration of solution CP
or MX in THF after neutralization with a small excess of NaOH/ methanol solution by aqueous 0.1 N HCl
solution
1H NMR spectra of both polymers are depicted
in Figure 2. It is obvious that the
areas of aromatic protons for both polymers are identical. That´s why it
was difficult to determine the amount of 9VA, 2VN and VBA in CP by 1H
NMR spectra.
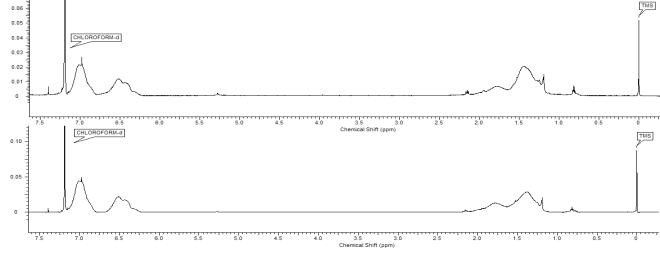
Figure 2. 1H NMR spectra of
CP (above) and MX (below).
Therefore we used the UV-absorption spectra for
quantifying the concentration of anthracene and naphthalene fragments into
polymer chain of CP using the system CH2Cl2:CH3OH
(9:1 v/v) as solvent. Figure 3 shows the absorption spectra
of 4 different concentrations of copolymers CP and MX in the spectral region
300-425 nm which has been used for determination of anthracene concentration.
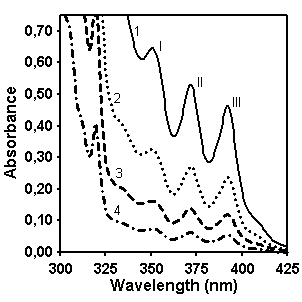
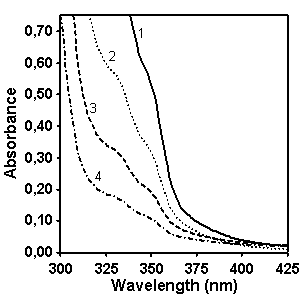
Figure 3. UV/vis absorption spectra of
CP (left, concentration for spectra 1 is 144.7 g/l, 2 – 72.35 g/l, 3 – 36.175
g/l, 4 – 18.087 g/l) and MX (right, concentration for spectra 1 is 220.5 g/l, 2
– 142.1 g/l, 3 – 71.05 g/l, 4 – 35.525 g/l).
It was found that the absorption maximum around 350 nm
(peak I on Figure 3) can be used for quantitative determination of anthracene
content due to the linear relationship between the intensity of this band and
concentration of polymer in solution.
Another spectral region (220-290 nm) and other
concentrations of copolymer CP in CH2Cl2:CH3OH
(9:1 v/v) solution have been chosen for quantifying content of naphthalene
groups in this copolymer (Figure 4).
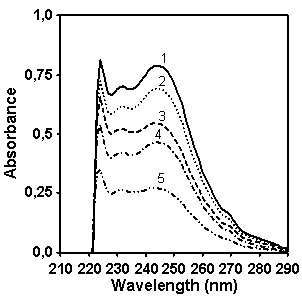
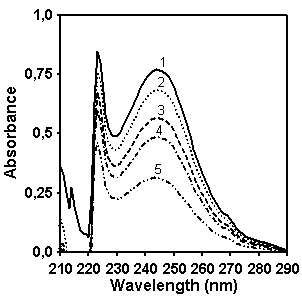
Figure 4. UV/vis absorption spectra of
CP (left, concentration for spectra 1 is 0.05 g/l, 2 – 0.043 g/l, 3 – 0.036 g/l,
4 – 0.029 g/l, 5 – 0.018 g/l) and MX (right, concentration for spectra is 0.049
g/l, 2 – 0.042 g/l, 3 – 0.035 g/l, 4 – 0.028 g/l, 5 – 0.0175 g/l).
In order to determine which wavelength should be used
for quantifying naphthalene fragment concentration in CP we compared the
absorption of copolymers CP and MX in the spectral region between 220 and 290
nm (Figure 5). It seems that the
intensity of absorption at 232 nm is the most distinctive feature in spectrum
of copolymer CP which allows to provide the quantitative estimation of
naphthalene contribution in the absorption. Figure 5 shows as well the absorption spectrum of model compound 2-ethylnaphtalene
which has been chosen for making a calibration graph for quantifying the
naphthalene content in copolymer CP.
As for determination of antracene content we used
another model compound – 9-methylanthracene. Table 1 summarizes data of spectrophotometrical evaluation of
naphthalene and anthracene fragments content in copolymer CP in comparison with
amount of corresponding monomers 2VN and 9VA which were used as starting
materials for copolymerization. It presents as well the data on content of
carboxyl groups in copolymer CP in comparison with concentration of VBA
introduced in copolymerization process.
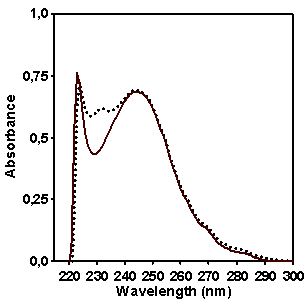
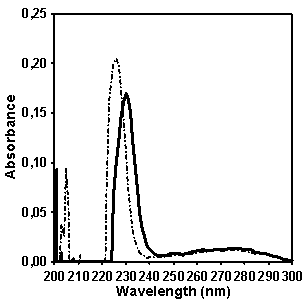
Figure 5. Comparison (left) and
difference (solid line, right) between the absorptions of CP (dotted line) and
MX (solid line, left) in comparison with 2-ethylnaphthalene (stick-dotted line)
as a model compound.
Table 1. Content of monomers 9VA, 2VN and VBA in
reaction mixture and in copolymer.
|
Monomers |
Mol. % in reaction mixture |
Mol. % in copolymer CP |
|
9VA |
0.1 |
0.0034 |
|
2VN |
0.37 |
0.037 |
|
VBA |
7.26 |
9.08 |
Conclusions
Free radical
copolymerization of styrene, 2-vinylnaphthalene, 9-vinylanthracene, and
4-vinylbenzoic acid has been reported.
Concentration of carboxy groups, as well as concentration of naphthalene and
anthracene derivatives in reaction mixture and determined concentration of
corresponding fragments in copolymer have been compared. It has been found that
copolymer contains about 30 times less of anthracene fragments, about 10 times
less of naphthalene fragments than amount of corresponding comonomers which
have been introduced into reaction.
References:
1.
Moser S.W.; Harder W.F.; Hurlbut C. R.; Kusner M. R. Radiat.
Phys. Chcm. 1993, 41, No. l/2. pp. 31-36.
2.
Grigor'eva V.I.; Gunder O.A.; Krasovitskii B.M.; Petrova I.B.
J. Appl. Spectros. 1968, 5, pp. 535-536.
3.
Barashkov N.N., Gunder O.A. Fluorescent Polymers, Ellis Horwood, UK, Chichester, 1994.
4.
Harmon J.P..; Gaynor J.F.; Taylor A.J.;
Radiat. Phys. Chcm. 1993, 41, No. l/2. pp. 153-159.
5.
Price, C.C.;
Halpern, B.D.; Voong, S. J. Polymer Sci. 1953,
11, pp 575-582.
6.
Loshaek S.; Broderick E. J. Polymer
Sci. 1959, 39, pp 241-247.
7.
Katz D. J. Polymer Sci. A. 1963,
1, pp. 1635-1643.
8.
Cherkasov A.S., Voldaikina K.G., Vysokomolek.
Soed., 1967,7, pp.175-179.
9. Tsubokawa N.; Kobayashi
K.; Sone Y.; Shimomura M. J. Macromol. Sci. A – Chem. 1988, 25, pp. 1475-1486.
10.
Prasath R.A.; Margarit-Puri K.; Klapper M. J. Appl. Polymer Sci. 2007,
103, pp. 2910–2919.