Danilov F.I., Vasilieva O.O., Smenova I.V., Protsenko
V.S.
Ukrainian State University of Chemical Technology,
Dnepropetrovsk, Ukraine
Iron electrodeposition from methanesulfonate
electrolyte
Electrodeposition of iron and its alloys has been
widely used for various engineering applications such as electrotypes,
electroforming, repairing worn and corroded machine parts, magnetics components
in computer and electronic industries, microelectromechanical systems and so on
[1-5]. Iron electrodeposits are usually obtained from acidic sulfate, chloride,
fluoroborate and sulfamate Fe(II) electrolytes, although weak-acid Fe(III)
baths [6] have been also reported.
Acidic iron electroplating baths are well studied;
they are highly productive and relatively simple as concerns their composition.
Nevertheless, acidic electrolytes for iron electrodeposition are rather
corrosive and toxic; therefore, development of novel acidic Fe(II) baths is an
important problem of modern electroplating.
Aqueous solutions of Fe(II) on the base of
methanesulfonic acid (MSA) seems to be an attractive and perspective
alternative to common iron electroplating baths as MSA is considered as a
"green acid" due to its environmental advantages [7]. MSA is known to
be far less corrosive and toxic than the usual minerals acids used in different
branches of industry. Methanesulfonate of various metals are highly soluble in water, the conductivity
of corresponding aqueous solutions is high. In addition, MSA is easily
biodegradable. Because of these advantages, electrochemical systems on the base
of MSA and its salts have been shown to be very promising for electroplating of different
metals and alloys [8-19].
Nonetheless, there are only several papers devoted to electrodeposition of
iron from methanesulfonate bath [20-22]. Thus, this question remains practically
unexplored. This communication is devoted to brief description of results of
our preliminary experiments relating to the problem involved, iron
electrodeposition from methanesulfonate electrolyte being compared with that
from "usual" sulfate electrolyte.
We have stated
that the deposition of iron is advisably to perform in the electrolytes containing
1.25 mol/dm3 Fe(CH3SO3)2 or 1.25
mol/dm3 FeSO4. There is no need to add some buffer or
conductive additives as well as surfactants to the baths.
The
optimal electrolyte pH seems to be in the range from 1.2 to 1.8. At lower pH
values (<1.2), the current efficiency of iron deposition decreases
dramatically due to the fact that hydrogen evolution reaction accelerates. At
higher pH values (>1.8), the rate of the chemical redox reaction between
dissolved oxygen and Fe(II) ions increases, and the latter process is highly
undesirable because the quality of deposits obtained deteriorates substantially.
As can be seen
in Figure 1, an increase in the current density results in an increase in the
current efficiency both in a sulfate bath and in a methanesulfonate one. For
the case of methanesulfonate electrolyte, current efficiency grows if the
current does not exceed 25 A/dm2, and then remains practically
constant. An increase in the bath temperature leads to a decrease in the
current efficiency at all employed current densities, this dependence being
more pronounced in case of sulfate electrolyte.
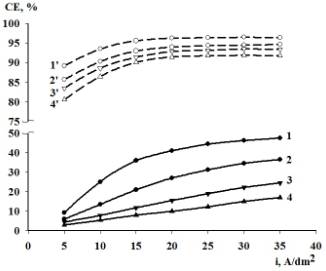
Figure 1.
Effect of current density on the current efficiency of iron deposition at bath
temperature (K): (1, 1Т) Ц 298; (2, 2Т) Ц 308; (3, 3Т) Ц 318; (4, 4Т) Ц 328.
Bath
composition: (1, 2, 3, 4) Ц1.25 mol/dm3 FeSO4; (1Т, 2Т,
3Т, 4Т) Ц 1.25 mol/dm3 Fe(CH3SO3)2;
pH 1.3
It should be
observed that the values of current efficiency in methanesulfonate
electrolyte are sufficiently larger than those in sulfate electrolyte, all
other conditions being kept identical. Such a feature is an essential advantage
of the methanesulfonate bath for iron electrodeposition in comparison with that
on the base of sulfate salts.
In
addition, the nature of anions exercises a significant influence upon the
surface appearance of the Fe coatings. Matt coatings deposits from sulfate bath
whereas bright deposits are obtained from methanesulfonate system.
It should be noted
that quite high values of current densities may be achieved in methanesulfonate
iron bath under consideration.
Microhardness
belongs to the most important properties of iron electrodeposits. As is shown
in Figure 2, the quantities of microhardness of coatings obtained from
methanesulfonate bath are greater than those in case of sulfate bath.
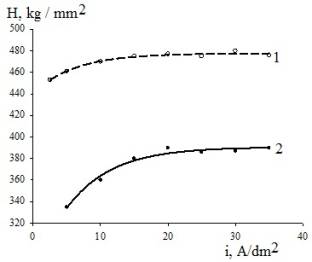
Figure 2.
Effect of current density on the microhardness of iron deposits (bath
temperature 298 K, pH 1.3)
Bath
composition: (1) Ц 1.25 mol/dm3 Fe(CH3SO3)2;
(2) Ц1.25 mol/dm3 FeSO4
Thus, methanesulfonate electrochemical systems for iron
electrodeposition seem to be highly attractive for practical use; they require
further comprehensive investigations.
References:
1. Y. Fujiwara, T. Nagayama, A. Nakae, M. Izaki, H. Enomoto, E.
Yamauchi, J. Electrochem. Soc. 143 (1996) 2584.
2. M. Panayotova, Surf. Coat. Technol. 124
(2000) 266.
3. A. Bai, C.-C. Hu, T.-C. Wen, Electrochim.
Acta 48 (2003) 2425.
4. F. Lallemand, L. Ricq, M. Wery, P. Berçot,
J. Pagetti, Appl. Surf. Sci. 228 (2004) 326.
5. N. Miyamoto, K. Yoshida, M. Matsuoka, J.
Tamaki, J. Electrochem. Soc. 151 (2004) C645.
6. F.I. Danilov, V.S. Protsenko, A.V. Ubiikon', Russ. J. Electrochem. 41 (2005)
1282.
7. M.D. Gernon, M. Wu, T. Buszta, P. Janney, Green Chem. 1 (1999) 127.
8. F.I. Danilov, I.V. Sknar, Yu.E. Sknar, Russ. J. Electrochem. 47 (2011)
1035.
9. N. M. Martyak, R. Seefeldt, Electrochim. Acta 49 (2004) 4303.
10. C.T.J. Low, F.C. Walsh, Electrochim. Acta 53 (2008) 5280.
11. C.T.J. Low, F.C. Walsh, Surf. Coat. Technol.
202 (2008) 1339.
12. H. Wang, M.
Pritzker, Electrochim. Acta 53 (2008) 2430.
13. C.S. Chen, C.C.
Wan, Y.Y. Wang, Trans. Inst. Metal Finish. 76 (2) (1998) 54.
14. F.I. Danilov, T.E. Butyrina, V.S. Protsenko, E.A. Vasil'eva, Russ. J. Appl. Chem. 83 (2010)
752.
15. F.I. Danilov, E.A. Vasil'eva, T.E. Butyrina, V.S. Protsenko, Prot. Met.
Phys. Chem. Surf. 46 (2010) 697.
16. F.I. Danilov, V.S. Protsenko, E.A. Vasil'eva, O.S. Kabat, Trans.
Inst. Metal Finish. 89 (3) (2011) 151.
17. G. Saravanan, S. Mohan, R.M. Gnanamuthu, J.
Vijayakumar, Surf. Eng. 24 (2008) 458.
18. S. Mohan, J. Vijayakumar, G. Saravanan, Surf. Eng. 25 (2009) 570.
19. V.S. Protsenko, A.A. Kityk, F.I. Danilov, J. Electroanal. Chem. 661
(2011) 213.
20. S.P. Sidelnikova, Yu.N. Petrov, Yu.S. Gorodetskii, Zashch. Met. 10 (1974) 187.
21. E.D. Pleshka, Surf. Eng.
Appl. Electrochem. 44 (2008) 92.
22. E.D. Pleshka, Surf. Eng.
Appl. Electrochem. 44 (2008) 264.