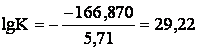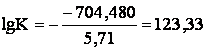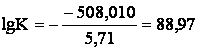D.ch.s. Skvortsov V.G.*, k.ch.s. Michailov V.I.*, k.ch.s. Ershov Ì.À.**,
k.ch.s. Koltsova Î.V.*, k.ch.s.
Pylchikova Yu.Yu.*,
Kamaev E.V.*,
Bagautdinov À.Ì.*
* The Chuvash State Pedagogical
University named I.Ya. Yakovlev
(ChGPU nam. I.Ya. Yakovlev),
Cheboksary, the Russian Federation
** Chuvash State Agricultural Academy
(ChSAA),
Cheboksary,
the Russian Federation
Inhibitory properties
of aliphatic amins and tHEIR borats in neutral
environments
The cause of metals’ corrosion is their thermodynamic instability in
various environments under the given external conditions.
Iron and steel corrosion in the
solutions close to neutral proceeds with oxygen depolarization [1-4]:
|
Fe ® Fe2+ + 2 e– (oxidation
of metal) O2
+ 2H2O + 4 e– ® 4OH– (restoration of the oxygen dissolved
in water) |
(1) (2) |
or totally:
|
2Fe + O2 + 2H2O ® 2Fe(OH)2. |
(3) |
In view of formation of 1 mol of substance we shall obtain:
|
Fe +
½ O2 + H2O ® Fe(OH)2. |
(3à) |
Change of Guibbs’ energy of reaction
(3a) under standard conditions is:
![]()
Constant of balance:
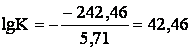 or
or ![]()
Calculations show that in the given corrosion environment iron is
thermodynamicaly unstable and under standard conditions of balance reaction
(3a) is moved much to the right.
The purpose of the our research is to study the influence of the nature
of alifatic amins (AA) and their compounds with a boric acid on corrosion electrochemical and corrosion fatigue behaviour of steel 10 (St. 10) and to establish an
opportunity of using them as inhibitor for protection of ferrous metals and
their alloys in neutral environments against corrosion.
In this connection we have studied the influence of ÀÀ: hexamethylendiamin H2N(CH2)6NH2
(HMDA),
ethylendiamin H2N(CH2)2NH2 (EDA),
diethylamin (C2H5)2NH2 (DEthA),
threbutylamin (C4H9)3N (TBA) and their
borats on
corrosion electrochemical behaviour and cyclic durability St. 10 in neutral
environments using the methods of gravimetry, removals potentionaly dinamical polarizing
curves and cyclic loadings. The borats of alifatic amins (BAA): BHMDA,
BEDA, BDEthA, BTBA have been obtained by the abore described techniques [5].
According to graviometric data we
studied rate of corrosion (r, g/m2×h), inhibitory
effect g and protection degree Z. Electrochemical measurements were carried out
on the potentiostat P-5848 in potentioanal dinamic mode of polarization. An electrode of comparison
was the silverclorid (cl.s.e.) one. Protective action of inhibitory additives were estimated in size
of density of an anode current (ia, mcÀ/sm2) in the
field of a passive condition at the potential of polarization j = +0,2 Â on cl.s.e. The basic characteristics of cyclic durability of
metal were defined on values of factor of a stock of cyclic durability ![]() on the basis of tests N = 4×106 cycles and cyclic durability N at s=±325,0 ÌPa. The distilled water (background1) and a solution containing 30 mg/l NaCl + 70 mg/l Na2SO4
(background2) were served as the corrosion active environment. Concentration of
additives was 2×10-2 mol/l. All measurements were made at a natural aeration and temperature 20 ± 0,2 îÑ. The table shows an average data of three parallel
measurements.
on the basis of tests N = 4×106 cycles and cyclic durability N at s=±325,0 ÌPa. The distilled water (background1) and a solution containing 30 mg/l NaCl + 70 mg/l Na2SO4
(background2) were served as the corrosion active environment. Concentration of
additives was 2×10-2 mol/l. All measurements were made at a natural aeration and temperature 20 ± 0,2 îÑ. The table shows an average data of three parallel
measurements.
The table. Influence AA and BAA on corrosion-electrochemical behavior
and the basic characteristics of cyclic durability of St. 10 in neutral
environments
|
The corrosion environment |
pH |
r×103, g/m2×h |
g |
Z, % |
ia, mcA/sm2 |
|
N, cycles |
|
Air |
327,0 |
5,0×106® |
|||||
|
Background1 |
6,6 |
39,37 |
1,00 |
0,00 |
– |
200,0 |
1,5×105 |
|
At presence |
|||||||
|
HMDA |
11,9 |
0,82 |
48,01 |
97,00 |
1,6 |
310,5 |
1,8×106 |
|
BHMDA |
8,9 |
0 |
¥ |
100,00 |
0,6 |
318,5 |
3,7×106 |
|
EDA |
11,5 |
0,99 |
39,77 |
97,49 |
2,0 |
304,5 |
1,6×106 |
|
BEDA |
8,9 |
0 |
¥ |
100,0 |
0,9 |
311,0 |
2,2×106 |
|
DEthA |
12,0 |
1,18 |
33,36 |
97,00 |
2,3 |
298,0 |
1,5×106 |
|
BDEtA |
8,9 |
0,42 |
93,74 |
98,93 |
1,8 |
306,5 |
1,7×106 |
|
TBA |
11,1 |
1,43 |
27,53 |
96,37 |
2,5 |
290,5 |
1,1×106 |
|
BTBA |
8,7 |
0,47 |
83,77 |
98,81 |
2,0 |
297,0 |
1,4×106 |
|
Background2 |
7,0 |
49,77 |
1,00 |
0,00 |
– |
186,0 |
1,4×105 |
|
At presence |
|||||||
|
HMDA |
11,9 |
2,04 |
24,40 |
95,90 |
2,0 |
281,5 |
7,5×105 |
|
BHMDA |
8,9 |
0,28 |
177,75 |
99,44 |
0,7 |
302,5 |
1,4×106 |
|
EDA |
11,5 |
3,17 |
15,70 |
93,63 |
2,4 |
273,5 |
6,5×105 |
|
BEDA |
8,9 |
0,33 |
150,82 |
99,34 |
1,5 |
289,5 |
9,0×105 |
|
DEthA |
12,0 |
3,67 |
13,56 |
92,63 |
2,6 |
262,5 |
6,0×105 |
|
BDEtA |
8,9 |
1,37 |
36,33 |
97,23 |
2,3 |
274,5 |
7,5×105 |
|
TBA |
11,1 |
4,42 |
11,26 |
91,12 |
3,0 |
258,0 |
5,0×105 |
|
BTBA |
8,7 |
1,47 |
33,86 |
97,05 |
2,5 |
261,0 |
5,6×105 |
On the table one can see, that steel intensively suffers from corrosion
in background electrolits. Clorid- and sulfate-ions noticeably accelerate corrosion
process. Introduction ÀÀ in corrosion environments essentially reduces value r and ia and raises
size ![]() and N, and BAA possess greater inhibitor
ability than corresponding ÀÀ. According
to their inhibitory abilities alifatic amins settle down in a following
decreasing order: HMDA > EDA > DEthA > TBA.
and N, and BAA possess greater inhibitor
ability than corresponding ÀÀ. According
to their inhibitory abilities alifatic amins settle down in a following
decreasing order: HMDA > EDA > DEthA > TBA.
Results of corrosion measurements, electrochemical researches and
corrosion fatigue tests perfectly correspond with each other.
Experimental data show, that the presence of amins and their borates
makes corrosion environment more alcalic (see the table). It leads in to the fact that sufficient
concentration of hydroacid-ions in electrolyte makes the passive oxide formation
possible without external anode polarization which is proved by the data of
thermodynamic calculations:
|
Fe + 2OH– « FeO + H2O + 2e–.
DG0=[–244,300+(–237,240)]–2(–157,335) = –166,870 kJoul/mol.
|
(4) |
|
3Fe + 8OH– « Fe3O4 + 4H2O
+ 8e–.
DG0=[–1014,200+4(–237,240)]–8(–157,335) = –704,480 kJoul/mol.
|
(5) |
|
2Fe + 6OH– « Fe2O3 + 3H2O
+ 6e–.
DG0=[–740,300+3(–237,240)]–6(–157,335) = –508,010 kJoul/mol.
|
(6) |
However it is necessary to note that there is no direct correlation between
inhibitory action of additives and the size of pH environments. Anticorrosive
properties of amins depend on their nature. Efficiency of amin action may be connected with
their ability to adsorption due to interaction of non divided electronic atomic
pairs of nitrogen with vacant d-orbital
atoms of iron. Monoamins have one center of adsorption and diamins –have two.
Therefore HMDA and EDA, containing two atoms of nitrogen, possess higher adsorption and
inhibitory characteristics in comparison with DEthA and TBA. It is necessary to
consider a spatial structure of amin molecules. The configurations of diethylamin
and threbutylamins, as well as ammonia, represent a trigonal pyramid, that
stericaly complicates adsorptions. In case of diamins the arrangement of nitrogen atoms admits their simultaneous
link to a surface of iron, i.e that the adsorption-active centers are not complicated
spatially. Therefore they form stronger links with metal that leads to the
strengthening of their inhibitory ability in comparison with monoamins.
According to [6] ocsoborats form dis soluble connections on metal. The increase of
inhibitory abilities BAA may be explained by the formation of a stronger protective
film the surface of a metal, formed by means of donor-acceptor ties through
n-doublets of nitrogen atom and borats-ions hemobsorbtion.
Thus, AA and BAA can be used as inhibitory additives for protection of ferrous metals and
their alloys against corrosion in neutral environments.
The literature:
1. Zhuk N.P. Course
of corrosion and protection of metals. – Moscow: Metallurgy, 1968. – 408 p.
2. Skorchelletti V.V. Theoretical basis of metals corrosion. –
Leningrad: Chemistry, 1973. – 264 p.
3. Ulig G.G., Revi R.U. Corrosion and struggle against it. Introduction
in a corrosion science and technics: Trans. from English / Editor. A.M.
Suhotins. – Leningrad: Chemistry, 1989. – 456 p.
4. Michailov V.I., Skvortsov V.G. Basis of corrosion and metal
protection. P.1. – Cheboksary: Chuvashgospeduniversitet, 2004. – 241 p.
5. Skvortsov V.G Interaction's of a boric acid and borats with organic
derivatives of ammonia // J. Inorgan. Chem. – 1986. – Vol. 31. – N 12. – P.
3163–3172.
6. Rosenfeld I.L. Ingibitory of corrosion. – Moscow: Chemistry, 1977. – 352 p.