Artur
Mantel1, Nikolay Barashkov2, Irina Irgibayeva1,
Anton Kiriy3 and Volodymyr Senkovskyy3
1L.N.
Gumilyov Eurasian National University, Astana, Kazakhstan
2Micro
Tracers, Inc., San Francisco, California, USA, Nikolay@microtracers.com
3Leibniz
Institute of Polymer Research, Dresden, Germany
Quaternary copolymer containing
styrene, hydroxystyrene and chromophoric monomers: synthesis, functionalization
and nanoagregation
Introduction
Nowadays there are many works dedicated to the
formation of polymer nanoparticles 1-3. Our interest in this sphere
is caused by the possibility of creating scintillator polymer materials able to
form nanoparticles and, consequently, to increase their quantum output. There
are hardly any works devoted to the nanosized organic scintillators, whereas
these materials are of great interest for science and technology. Our attention
was attracted by the works by E. W. Meijer et al., where for the formation of
nanoaggregates were used functional groups of the 2-ureido-pyrimidinone (UPy),
capable of forming between each other a quadruple hydrogen bond and lead to the
collapsing of single chains in a solution 4-6. In the suggested work
we describe the production of a copolymer containing the specified amounts of
naphthalene and anthracene chromophore links, its functionalizing by means of a
UPy-fragment, studies of the nanoaggregation ability and the influence of this
aggregation on the fluorescence spectrum.
Experimental
Materials.
All reagents were purchased from the Aldrich Chemical Co.
2-vinylnaphthalene (2VN), 9-vinylnaphthalene (9VA) and styrene (Sty) were
purified from stabilizator, products of oligomerization by silica column with
hexane as eluent; 2-vinylnaphthalene(2VN), 9-vinylanthracene (9VA) then were
dried out in vacuum in presence of phosphorous pentoxide to remove of traces of
water and solvents and characterized by 1H NMR. 4-Acetoxystyrene
(4AS) (97%, Aldrich) was distilled under reduced pressure prior to use;
2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidine was prepared from (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl (TEMPO)
and (1-bromoethyl)benzene by the method presented below.
Instrumentation.
Gel permeation chromatography (GPC) was used to determine the molecular
weights and molecular weight distributions, Mw/Mn, of polymer samples with
respect to polystyrene standards. The system configuration: THF with flow rate
1.0 ml/min. HPLC-Pump, Ser. 1200, Agilent Technology; ETA-2020 – RI .1H
NMR spectra were collected on the device «Bruker Bio Spin» (1H 500
MHz, 20 0Ñ, solvent -CDCl3). Scanning electron microscopy
was carried out using Ultra 55 (Carl Zeiss SMT, Jena, Germany).UV/vis
absorption spectra were measured on Perkin Elmer Lambda 800 spectrophotometer.
Copolymerization of styrene
2-vinylnaphtalene, 9-vinylanthracene and 4-acetoxystyrene. Mixture of 4-Acetoxystyrene (13.35 mmol,
2.16594g), TEMPO (0.04 mmol, 0.0062g) and
2,2,6,6-tetramethyl-1-(1-phenylethoxy)piperidine (0.399 mmol, 0.1044g) was
dissolved in 8 ml (49.08 mmol, 5.11422g) of styrene. Mixture was divided into
two equal parts. One of them was placed in a flask containing 9-vinylanthracene
(0.5 mmol, 0.10235g) and 2-vinylnaphthalene (1.67 mmol, 0.25756g). Second part
was operated without addition of 9-vinylanthracene and 2-vinylnaphthalene.
Traces of oxygen were removed from both parts by three freeze–pump–thaw cycles.
Flasks were flushed with dry argon. Both mixtures were stirred overnight at the
temperature 130 0C. Prepared copolymers were dissolved in the system
CH2Cl2:CH3OH 9:1 and precipitated in methanol
three times. Polymers were dried to constant weight in vacuum at temperature 70
0C and characterized by 1H NMR and UV/vis absorption
spectroscopy.
Synthesis of OH-group
containing copolymers CPolOH and
MPolOH). A. Removal of Acetyl Groups by Hydrazinolysis (Deacetylation) from
Precursor Copolymers CPOL and MPOL) (General Procedure). In a 1 L round flask 2 g of (3.35 mmol of
acetyl groups) copolymer CPOL were dissolved in 250 mL of dioxane. The solution
was stirred for 15 min, and 1 mL (20.6 mmol) of hydrazine monohydrate was added
slowly with a syringe. After additional stirring for 24 h the solution was
concentrated. The polymer was precipitated in 400 mL of water, filtered, and
dried at 40 °C under vacuum. This procedure was repeated twice in order to
remove all low molecular byproducts and impurities. After drying in vacuum in
presence of phosphorous pentoxide to remove of traces of water 1,53 g of CPolOH
was obtained as a white-green powder. According to 1H NMR
spectroscopy all acetyl groups have been quantitatively removed. Similar
procedure was used for removal of acetyl groups from copolymer MPOL.
Synthesis of Upy-group
containing copolymer CPolOH.
1.5 g of copolymer CPolOH was dissolved in dry toluene
in argon atmosphere. The solution was turbid. In this solution was added dry
triethylamine to make solution clear.
2.1 g of
2(6-isocyanatohexylaminocarbonylamino)-6-methyl-4[1H]pyrimidinone7
was dissolved in hot toluene in argon atmosphere, and then cooled to room
temperature. Prepared solution was introduced slowly in solution of CPolOH
throw filter with pore size 0.2 μm by stirring. When solution comes to
turbid several drops of triethylamine were added to make solution clear. After
additional stirring for 24 h the solvent was evaporated, polymer dissolved in
acetone, centrifuged and precipitated in hexane throw filter with pore size 0.2
μm. This procedure is repeated three times and resulting powder (CUPOL)
dried at 80 °C under vacuum and characterized by 1H NMR.
Results
and Discussion
Figure 1 shows the polymerization process and
chemical structure of prepared quaternary copolymer (CPOL). In case when l=n=0, the corresponding copolymer
without chromophore fragments (MPOL) has been prepared.
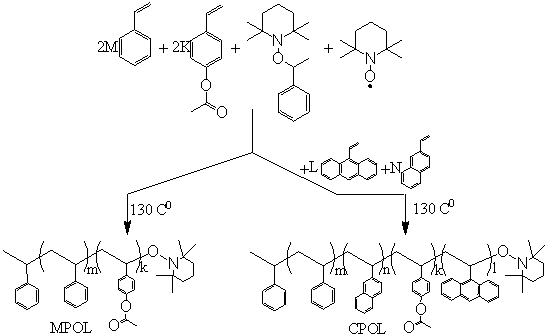
Figure
1. Copolymerisation of styrene
2-vinylnaphtalene, 9-vinylanthracene and 4-acetoxystyrene.
According to GPC data, the Mw of copolymers CPOL and
MPOL are 15200 and 17300, respectively. The determined molecular weight
distributions have been equal to 1.27 and 1.38 for CPOL and MPOL, accordingly.
The amount of 4-AS in copolymers has been determined by 1H NMR
spectroscopy.
Concentration of anthracene and naphthalene
fragments in copolymer CPOL has been determined by means of the UV-absorption
spectroscopy using the mixture CH2Cl2:CH3OH
(9:1 v/v) as a solvent (Table 1).
Table 1. Content of monomers 9VA, 2VN and 4AS in reaction mixture and in copolymer.
|
Monomer |
Mol. % in reaction mixture |
Mol. % in copolymer CPOL |
|
Styrene |
73.5 |
79.03 |
|
Acetoxystyrene |
20 |
17.58 |
|
9-vinylanthracene |
1.5 |
0.31 |
|
2-Vinylnaphtalene |
5 |
3.08 |
Nanoagregation of Upy-group
containing copolymers.
Table 2 summarizes data about the ratios between a
good solvent (dichloromethane) and bad solvent (methanol) which were combined
together for making samples of modified copolymer CUPOL, which has Upy-group, and unmodified copolymer MPOL with a
starting concentration Cstart. These solvent systems were used for
scanning electron microscopy analysis (SEM)
Table 2. Concentrations and solvent systems for
solutions of MPOL and CUPOL.
|
Copo-lymer |
Cstart (g/l) |
System 1 |
System 2 |
System 3 |
|||||
|
|
CH2Cl2 (ml) |
MeOH (ml) |
CH2Cl2 (ml) |
MeOH (ml) |
CH2Cl2 (ml) |
MeOH (ml) |
|
|
|
MPOL |
4.44 |
0.1 |
1.5 |
0.4 |
1.5 |
1.6 |
0 |
||
|
CUPOL |
4.61 |
0.1 |
1.5 |
0.4 |
1.5 |
1.6 |
0 |
||
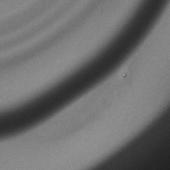
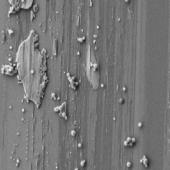
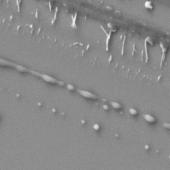
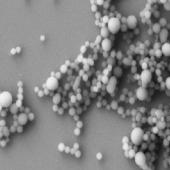
Samples for SEM analysis were prepared by placing 10
μl of each from all 6 combinations of polymer/solvent presented in Table 2
on the surface of a silica plate, following by full evaporation of the solvents
(Table 3).
Table 3. SEM pictures of samples prepared from all
6 combinations of polymer/solvent presented in Table 2
|
|
System 1 |
System 2 |
System 3 |
|
MPOL |
|
|
|
|
CUPOL |
|
|
|
Presented data indicate that the average size
nanoparticles prepared from copolymer CUPOL/System 2 and CUPOL/System 3 is
about 90 nm.
Figure 2 illustrates data related to absorbance and
fluorescence spectra of copolymer CUPOL in solutions of THF (A, B, C) and
mixture of THF/MeOH (68:32 v/v) (D, E, F). The choice of the solvent systems
was related to our intention to observe the intra- and interpolymer
interactions as a function of solvent-induced coil changes. It is known8
that for naphthalene-containing polymers the ratio of monomer to excimer
fluorescence (Im / Ie) is very dependent on solvent
(and/or coil density). The general observation is that a poor solvent (mixture
THF/MeOH) enhances the excimer fluorescence (420-475 for naphthalene fragments)
at the expense of the monomer fluorescence (310-340 nm). In these solvents, the
coil density is increased such that naphthalene-naphtalene separations are
decreased, which in turn leads to a higher density of excimer-forming sites. In
fact, the comparison of fluorescence spectra of copolymer CUPOL which has no
anthracene units ( l=0 in structure
presented in Figure 1) dissolved in THF and THF/MeOH shows the ratios Im
/ Ie equal to 40.0 and 32.9, respectively (excitation at 285 nm).
This observation helps to explain the differences in the intensity of fluorescence
presented in Figure 2B and 2E where the
same excitation wavelength has been used. Taking the intensity of emission at
420 nm as a parameter for monitoring, it is easy to estimate that due to
contribution of excimer emission of naphtalene fragments in this spectral
region the increase in the intensity measured for THF/MeOH solution compared to
THF solution is changing from 12.3% to 23.0% when the concentration of
copolymer CUPOL has been decreased from 7.5 to 1.9 g/L. The nature of this
concentration dependence is not completely clear because it is expected that
the ability to form excimer sites is usually enhances with increasing concentration
of chromophore groups. It is interesting that increasing excitation wavelength
to 391 nm (Figures 2C and 2F) leads to the opposite effect in terms of
intensity of fluorescence: solution on THF has a higher intensity that solution
in mixture THF/MeOH. That observation can be considered as a additional
confirmation of provided explanation related to the contribution of excimer
emission of naphtalene fragments which is excited by wavelength 285 nm, but not
wavelength 391 nm.
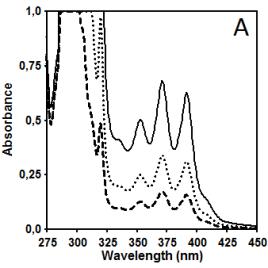
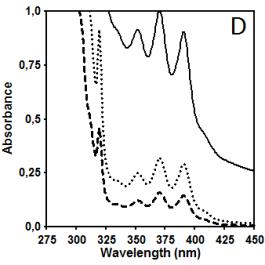
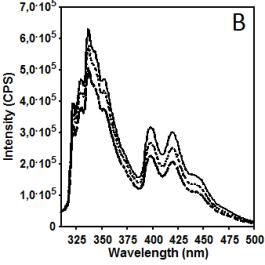
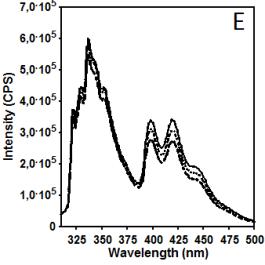
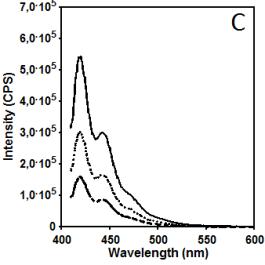
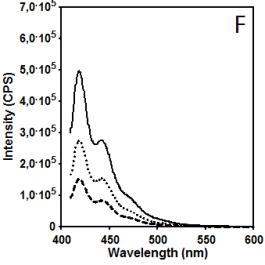
Figure 2. Absorbance (A, D) and fluorescence spectra
of CUPOL in solutions of THF (A,B,C) and mixture of THF/MeOH (D, E, F) at excitation 285 nm (B,E)
and 391 nm (C,F) at three different
concentrations: 7.5 g/L (solid line); 3.7 g/L (dotted line) and 1.9 g/L
(dash-dotted line).
Conclusions
Synthesis of copolymers containing styrene,
9-vinylanthracene, 2-vinylnaphthalene and 4-hydroxystyrene, and
fictionalization of them by 2-ureido-pirimidinone has been reported.
Aggregation ability of the functionalized copolymer with formation of
nanoparticles has been investigated. Absorbance and fluorescence properties of
the functionalized copolymer in two different solvent systems have been studied
and interpreted in terms of specific ability of naphthalene-containing polymer
dissolved in a poor solvent to enhance the excimer fluorescence at the expense
of the monomer component.
References
(1) Seo, M.; Beck,
B.J.; Paulusse, J.M.J.; Hawker, C.J.; Kim, S.Y. Macromolecules 2008, 41, 6413.
(2) Bertin, P.A.; Gibbs, J.M.; Shen, C.K.F.;
Thaxton, C.S.; Russin, W.A.; Mirkin, C.A.; Nguyen, S.T. J. Am. Chem. Soc. 2006, 128, 4168.
(3) Pochan, D.J.; Chen, Z.; Cui, H.; Hales, K.; Qi,
K.; Wooley, K.L. Science 2004, 306, 94.
(4) Foster, E.J.;
Berda, E.B.; Meijer, E.W. J. Am. Chem.
Soc. 2009, 131, 6964.
(5) Beijer, F.H.;
Sijbesma, R.P.; Kooijman, H.; Spek, A.L.; Meijer, E.W. J. Am. Chem. Soc. 1998,
120, 6761.
(6) Feldman, K.E.;
Kade, M.J.; de Greef, Ò.F.A.; Meijer, E.W.;
Kramer, E.J.; Hawker, C.J. Macromolecules 2008, 41, 4694.
(7) Keizer, H.M.;
Kessel, R.; Sijbesma, R.P.; Meijer, E.W. Polymer
2003, 44, 5505.
(8) Cao, T.; Webber, S.E. Macromolecules 1989, 22, 646.