Õèìèÿ/8. Êèíåòèêà è êàòàëèç
A.S. Savenkov, N.Yu. Masalitina, V.V. Rossikhin
National
Technical University "Kharkiv Polytechnic Institute"
OXIDATION OF AMMONIA:
EXPERIMENTAL STUDIES AND MATHEMATICAL MODELLING
Currently, the only industrial process for producing
nitric acid is the catalytic oxidation of ammonia with oxygen from the air,
followed by absorption of nitrogen oxides produced by water. The stage of NH3
change ratio largely determines the performance of all nitric acid production.
The conditions and the quality of its implementation determine the consumption
indices of NH3, input and losses of platinum group metals (PGM) and
energetic capabilities of process conditions.
The studies conducted [1–4] justify the assumption
about the mechanism of the ammonia catalytic oxidation on platinoid gauzes,
give a kinetic model of the reaction, and allow us to determine the conditions
of maximum NO output. As the analysis of the bibliography and our experimental
research has shown, to ensure the maximum NO selectivity and high intensity of
the process it is necessary to maintain external diffusion conditions. At low
NH3 concentrations
of the catalyst surface, which is typical for external diffusion conditions,
the rates of its oxidation to N2O and N2 decrease dramatically
and NH3 selective oxidation to NO takes place [1–4].
One of the shortcomings of previous studies and
previously-developed models is the lack of data on the nitrogen oxide formation
developed as a byproduct by NH3 catalytic oxidation in the HNO3
manufacture process [5–7]. Nitrogen oxide passes through the remaining stages
of the HNO3 production technology unchanged and then is released
into the atmosphere. The facilities for the nitric acid manufacture in the CIS
countries currently emit about 6.0–7.5 kg of N2O per year per ton of
HNO3. According to the latest research N2O is a strong
greenhouse gas, whose global warming potential is 310 times greater than the
one of CO2 [8].
Results and
discussion. To create a mathematical model of the ammonia oxidation
to NO with due consideration of N2- and N2O-synthesis, a
complex of physicochemical, kinetic and technological research has been carried
out.
The essential results of the studies which where
carried out at the flow-type facility, and which determined the degree of
ammonia oxidation into nitrogen (II) oxide NO and nitrogen (I) oxide N2O,
where the catalyst PGM-gauzes composition – Pt – 90 %, Rh – 10%; wire diameter
of catalyst gauzes is dg =
0.072×10–3
m. The analysis of the results of the studies shows that along with a high
NO output, a simultaneous oxidation of ammonia to N2O and N2
is observed, though the rates of these processes are low. At the same time the
conversion of NO into N2 was revealed.
Taking to account the formation of nitrogen (I) oxide
for a PGM-catalyst, the stoichiometric basis of ammonia oxidation reaction
routes, has the following form:
4NH3 + 5O2 ![]() 4NO + 6H2O (1)
4NO + 6H2O (1)
4NH3 + 4O2 ![]() 2N2O + 6H2O (2)
2N2O + 6H2O (2)
4NH3 + 3O2 ![]() 2N2 + 6H2O (3)
2N2 + 6H2O (3)
2NO ![]() N2
+ O2 (4)
N2
+ O2 (4)
Modelling of the process is reduced
to a mathematical description of the process in the catalyst bed considering
the assumed reaction mechanism. At a high temperature oxygen on the platinum
surface is mainly in the dissociated state. The ammonia adsorption, as well as
its dissociation and formation of intermediates interacting with each other,
leads to the formation of NO, N2O, N2, Í2Î and
desorption from the free catalyst surface at 973–1193 K.
In the process of catalysis the
ammonia acts as an electron-donor, and oxygen – as an electron-acceptor.
Adsorbed Î2 molecules attach electrons from the catalyst surface and
turn into oxygen surface atoms (step 1). On step 2 NH3 molecule donates
electrons from the catalyst surface which is not covered with oxygen, N-H bonds
are thus weakening and the imido-particles are being formed. On the catalyst
surface coated with oxygen, the ammonia forms particles of the imide-type (step
3), which are then recombining and form the nitrogen (step 7). Further addition
of oxygen to the imide (steps 4, 5) leads to the formation of NO. Steps 6, 8,
and 9 are connected with N2O, N2 and Í2Î
formation.
The proposed detailed mechanism of the process has
been used for deriving kinetic equations, which describe the reactions on the
catalyst surface and relate to the formation of O, N2O, N2.
The kinetic model of the process of ammonia oxidation
for routes I–IV can be written as:
![]() ,
, ![]() ,
, ![]() ,
, ![]() (5)
(5)
where r1, r2, r3, r4 –
rates of the routes I–IV; k1, k2, k3, k4
– kinetic rate constants of the routes I–IV.
The kinetic equations of the ammonia consumption and
NO formation reactions have the following form:
![]()
![]() (6)
(6)
The equations (5–6) were
used for description of the ammonia oxidation process on the catalyst
PGM-gauzes.
The process in the catalyst bed for
external diffusion condition is characterized by a large temperature gradient.
At a small distance - a few centimeters in front of the catalyst – the gas is
heated by the amount of the adiabatic heating of the reaction mixture from 473
to 1173 K. This can lead to significant longitudinal heat and mass transfer.
However, the effective coefficients of longitudinal diffusion and thermal
conductivity of the gas phase are small and longitudinal heat transfer is
carried out mainly along the solid skeleton of the catalyst bed. When
describing the process the influence of the flows arising in non-isothermal
boundary layer of multi-component mixture - Stefan flow, thermal diffusion, as
well as diffusion thermal conductivity can be neglected, since the volume of
the reaction mixture does not vary by more than 10%, and it is highly diluted
with inert gas N2; molecular weights of components and, thus, their
diffusion coefficients differ insignificantly [7].The
temperature of the catalyst in the bed of PGM-gauzes is assumed to be constant
and is determined by the adiabatic heating of the coming reaction mixture. On
this basis, to describe the process, a model of plug-flow for the gas phase is
accepted with the use of material balance equations for each component.
The material
balance equation of the process occurring at a significant influence of
external mass transfer to the i-component
is as follows:
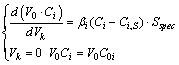 , (7)
, (7)
where i = 1, 2, 3, 4, 5, 6 – the following components respectively: NH3,
O2, NO, H2O, N2O, N2; V0 × Ci – molar amount of the i-component in the catalyst layer; Vk – the volume of the
catalyst layer; Ci, Ci,S – the current
concentration of i-component in the
flow of reactants and on the surface respectively; C0i – the initial concentration rate of ammonia and
oxygen in the reaction mixture; b
– mass transfer coefficient between the catalyst surface and the flow of the
reaction mixture; Sspec –
specific geometric surface of catalyst PGM-gauzes per unit of volume.
For ammonia and oxygen the equation (8) has
the following form:
![]() , (8)
, (8)
where i = 1, 2; xi – the degree of conversion of ammonia and oxygen,
mole fraction; y0i – the
initial mole fraction of ammonia in the mixture; yi, yiS – the current mole fraction of ammonia and
oxygen in the volume and on the surface of the catalyst; t – the
contact time, s; ![]() ;
; ![]() – volumetric flow
rate of the gas mixture under normal conditions (T = 273 K, p = 0.098
MPa).
– volumetric flow
rate of the gas mixture under normal conditions (T = 273 K, p = 0.098
MPa).
Similar equations were obtained for all components in
the reaction mixture. A mathematical description of ammonia oxidation process
in the catalyst layer has the following form:
![]() (9)
(9)
with boundary conditions t
= 0, xi =0, xk = 0; where ![]() = 3, 4, 5, 6; xê – mole number of NO, H2O, N2O,
N2 formed from one mole of ammonia.
= 3, 4, 5, 6; xê – mole number of NO, H2O, N2O,
N2 formed from one mole of ammonia.
The
mole fraction of nitrogen y6 was calculated according to the
equation:
![]() . (10)
. (10)
The current
concentration of components in the mixture was calculated according to the mole
fractions by the formulas:
![]() , (11)
, (11)
where i = 1, 2;
![]() , (12)
, (12)
where ![]() = 3, 4, 5, 6; N – the current number of moles of the
reaction mixture, which is equal to the current sum of the moles of each
component.
= 3, 4, 5, 6; N – the current number of moles of the
reaction mixture, which is equal to the current sum of the moles of each
component.
Concentration of
substances on the catalyst surface was determined according to the following
equations:
![]() ,
, ![]() ,
, ![]() (13)
(13)
where i = 1, 2, 3, 4, 5, 6; T0 – temperature of the mixture in the gas volume, K; TS – the catalyst surface temperature, Ê; rj – the
reaction rate on the j-route
(reactions 1–4); ![]() – the thermal
effect of the reaction on the j-route;
– the thermal
effect of the reaction on the j-route;
![]() – the
coefficient of heat transfer from the gauzes to the gas stream, kJ /(m2·s·Ê).
– the
coefficient of heat transfer from the gauzes to the gas stream, kJ /(m2·s·Ê).
The system of equations (7–13) is
the mathematical model of external diffusion processes of ammonia oxidation on
catalyst PGM-gauzes.
The degree of NH3 oxidation on
PGM-catalysts was calculated according to the developed mathematical model. For
calculating this process it is necessary to know the coefficients of heat and
mass transfer from the gas flow to the catalyst, as well as the physical and
chemical properties of the mixture, depending on the temperature, pressure and
composition of the mixture.
By solving the inverse problem of chemical kinetics,
using the experimental data, we determined reactions
rates constants connected with the formation of NO (k1), N2O
(k2), N2 (k 3) and N2, resulting
from the nitrogen (²²) oxide
decomposition (k4), and the concentrations of the substances on
the catalyst surface [12–14]. The comparison of calculated and experimental
conversion coefficients shows good agreement, the difference is not more than
1–3%.
Conclusions. A
mathematical model of the oxidation process of NH3, considering the
physical and chemical properties of the ammonia-air mixture and nitrous gas has
been developed. The rate constants and their temperature dependence have been
determined. This will allow to determine the optimal process conditions of NH3
oxidation on the PGM-catalyst at different pressures in a wide range of process
parameters, as well as to determined the amount of nitrogen oxides N2O
formed. On the basis of the developed model, a program for calculating the NH3
oxidation reactor using PGM-catalysts of different geometric profile has been
created. During the operation process, the volume and the surface of the
catalyst are changed. Thus the mathematical model for the rates of ammonia
oxidation steps per unit mass of PGM-catalyst has been developed.
REFERENCES
[1]
Savenkov À.S.: Catalyticheskie svoistva poverhnosti platiny v reakcii îkislenia
amiaka i matematicheskoe modelirovanie processa [in:] A. Loboiko (Ed.),
Catalyticheskie i massoobmenye processy pod davleniem v technologii
neorganicheskih veshchestv, Kharkov, Osnova 1993, 46-63.
[2]
Savenkov À.S., Beskov V.S.: Êinetica processa okislenia ammiaka na platinovom
katalizatore pod davleniem [in:] V.I.Atroshchenko (Ed.), Êinetica
geterogenno-kataliticheskih processov pod davleniem, Kharkov, Vishcha shkola
1974, 67–83.
[3] Vyatkin
Ju.L., Savenkov À.S., Beskov V.S.: Khimicheskaja Promishlennost, 1979, 10, 618.
[4]
Beskov V.S., Vyatkin Ju.L., Savenkov À.S.: Teoreticheskie Osnovy Khimicheskoi
Tehnonologii, 1980, 14, 442.
[5]
Beskov V.S., Êusnkov À.P., Novikov E.À. et al.: Khimicheskaja
Promyshlennost, 1977, 9, 691.
[6]
Savenkov À.S., Beskov V.S., Vyatkin Ju.L.: Proceed. 10th Annual
Conference "Ñhemreactor-10", Ukraine, Kharkov, 1992, 339.
[7] Beskov V.S., Brushtein Å.À., Golovnia Å.Â.,
Vanchurin V.I.:
Catalysis in Industry, 2008, 2, 31.
[8]
Pérez-Ramírez J.: Applied Catalysis B: Environmental, 2003, 44, 117.
[9]
Cybulski A., Moulijn J.A., Stankiewicz A. Novel. Concepts in Catalysis and Chemical
Reactors. – WILEY-VCH, 2010, 372 p.
[10] D.A. Hickman , L.D. Schmidt: Ind. Eng. Chem. Res., 1991, 30(1), 50.
[11]
Vargaftik N.B. Spravochnik po teplophysicheskim svoistvam gasov i zhidkostei,
Nauka, Moscow, 1972.
[12] Bliznjuk Î.N., Savenkov À.S.,
Masalitina N.Yu., Ogurtsov A.N.: Visnyk NTU "KPI", 2010, 13, 8.
[13] Bliznjuk Î.N., Savenkov À.S.,
Masalitina N.Yu., Ogurtsov A.N.: Khimichna Promyshlovist Ukraine, 2010, 3(98), 3.
[14] Bliznjuk Î.N., Savenkov À.S., Ogurtsov
A.N., Chlopitskii A.A.: Voprosy Khimii i Khimichnoi technologii, 2012, 6, 92.