Chemistry and Chemical Technologies/4. Chemical and
Pharmaceutical Industry
Dr. Belakhov V.1, Dr. Botoshansky M.1,
Dr. Goldstein D.2, Dr.Sc., Prof. Ionin B.I.3
Towards application of novel
stabilized derivatives of vitamin C in cosmetics: synthesis, crystal structure
and biological evaluation
of 2-acyl(aryl)-3-phosphoryl
derivatives of ascorbic acid
1Schulich Faculty of Chemistry, Technion – Israel
Institute of Technology
Technion City, Haifa 32000, Israel; 2Tagra Biotechnologies Ltd.,
P.O. Box 8213, 8 Hamlacha Str., Netanya 42293, Israel; 3Department
of Organic Chemistry, Saint-Petersburg State Technological Institute 26
Moscowsky Str., Saint-Petersburg, 190013, Russia
Ascorbic acid (AsA, vitamin C) is a
vital nutrient for human and has many important functions in its organism. AsA
is essential for collagen synthesis and helps maintain the integrity of
substances of mesenchymal origin, such as connective tissue, osteoid tissue,
and dentin [1, 2]. AsA has been used in recent years as an active ingredient of
cosmetics [3–5]. Due to its antioxidant properties, it is considered to confer
both antioxidant and photoprotection to skin against free radical attack and UV
ray damage [6]. The formulation of pure AsA into a final product, however,
presents serious difficulties because it is easily oxidized. In recent years,
in order to overcome the problem of the lack of stability of ascorbic acid in
its pure form, various semi-synthetic stable derivatives of AsA were prepared
by several research groups [7–11].
In
this report we presented synthesis and biological evaluation of novel 2-acyl
dialkyl(aryl)-3-phosphoryl derivatives 1 of AsA, crystal structure of
one of the intermediate compound 2, and biological evaluation of
synthesized derivatives 1.
Experimental Chemical
Part
1H NMR spectra were
recorded on a Bruker AvanceTM 500 spectrometer, and chemical shifts
reported (in ppm) are relative to internal Me4Si (d =0.0) with CDCl3 as the solvent, and
to HOD (d =4.63) with D2O
as the solvent. 13C NMR spectra were recorded on a Bruker AvanceTM
500 spectrometer at 125.8 MHz, and the chemical shifts reported (in ppm)
relative to the residual solvent signal for CDCl3 (d =77.00), or to external sodium
2,2-dimethyl-2-silapentane sulfonate (d =0.0) for D2O
as the solvent. Mass spectra analysis were obtained either on a Bruker Daltonix
Apex 3 mass spectrometer under electron spray ionization (ESI), or by a TSQ-70B
mass spectrometer (Finnigan Mat). Reactions were monitored by TLC on Silica Gel
60 F254 (0.25 mm, Merck), and spots were visualized by charring with
a yellow solution containing (NH4)Mo7O24.4H2O
(120 g) and (NH4)2Ce(NO3)6 (5 g) in
10% H2SO4 (800 mL). Flash column chromatography was
performed on Silica Gel 60 (70-230 mesh). All reactions were carried out under
an argon atmosphere with anhydrous solvents, unless otherwise noted. All
chemicals unless otherwise stated, were obtained from commercial sources.
Experimental Biological
Part
In
order to evaluate the effect of prepared derivatives of AsA 1 on
collagen synthesis, cultured human foreskin fibroblasts were placed in 24-well
microculture plates in DMEM supplemented with 10% fetal calf serum containing
100 mg/ml b-aminopropionitrile, 10 mCi [2,3-3H]proline,
in the presence of either AsA (positive control) or the prepared derivatives of
AsA in various concentrations, e.g. from 1mM to 50 mM. The cultures were
incubated for 24 hours. The [2,3-3H]proline incorporation into
pepsine-resistant salt precipitated extracellular collagen was determined and
used as an index of efficiency of the collagen synthesis.
Experimental
Crystallographic Part
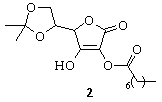
2-Capryloyl-5,6-O-isopropylidene-L-ascorbic acid (2) was
recrystallized from ethyl acetate-hexane (1:1). Intensity data from crystals of
compound (2) were collected at 293(2) K on a Nonius KappaCCD
diffractometer. Data collection was made by application of the Collect program
Nonius-2006 [12]. Data reduction and space group determination were performed
using the DENZO HKL-2000 program [13]. The SHELXS-97 program was used for
crystal structure solution by application of direct methods [14]. The SHELXL-97
program was used for refinement by full-matrix least squares [15]. The molecular
graphics was performed using TEXSAN program (TEXSAN.
Molecular Structure Corporation, 1999).
Results and Discussion
The
preparation of title compounds 1 was accomplished in four chemical
steps. At the first step, at 0°C 5,6-isopropylidene derivative of AsA was obtained in
almost quantitative yield by the treatment with dry acetone excess in the
presence of CuSO4. Regioselective acylation at position 2 of this
compound was carried out by using corresponding acyl chlorides in anhydrous
pyridine in the presence of catalyst (4-dimethylaminopyridine) at 0°C.
Phosphorylation of protected intermediate with dialkyl(aryl)phosphochloridates
in anhydrous pyridine at –5°C afforded derivatives of AsA with phosphate group at the position 3. At the last step, deprotection of
5,6-isopropylidene-3-dialkyl(aryl) phosphoryl derivatives of AsA was carried
out in mild conditions with dilute HCl at 0°C to furnish the final
products 1 with excellent purity and high yields [16].
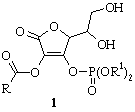
R = Me, Et, Ph; R1 = (CH2)nMe
[n = 6, 14]
Biological tests indicated that studied
derivatives of AsA 1 indicated higher level of activity by stimulation
of the collagen synthesis in human foreskin fibroblasts in comparison with AsA.
During the last two decades an interest in crystal
structures of derivatives of L-ascorbic acid has greatly
increased, and interestingly according to Cambridge Structural Database [17]
out of 38 known structures more than 60% were established starting 1999. This
can be explained by the fact that application of L-ascorbic acid and its
derivatives in medicine [1, 2, 6], nutritional industry [18-20] and cosmetics
[21-23] has significantly increased. In recent years various research groups
have showed that L-ascorbic acid and its derivatives can be considered as
potential anticancer [24-27], antitumor [28, 29], antiviral [30-32] and anti-inflammatory agents [33].
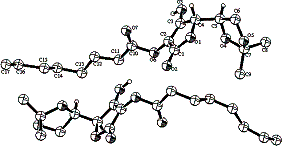
Structure of the compound 2,
obtained by means of Õ-ray analysis contains two independent very
similar molecules in an asymmetric unit of space group P21. In one
of the molecules capryolyl substituent at the C-2 position is partly
disordered. The main difference between the molecules was found in relative
conformation of 1,3-dioxalane rings. At the first and the second molecules
these rings have inverted boat conformation. As an evidence of this fact, a
deviation from the planarity and torsion angles have close absolute values, but
with opposite sings. It was found also that dihydrofuranone rings are strictly planar,
and in addition a deviation from the mean plane has not increased over 0.0047
Å in both molecules. Relatively strong intermolecular hydrogen bonds
interaction includes a hydroxyl group at the position of C(3) and oxygen atoms
in the position of C(1) of neighbored furanone rings. Thus, H…O distances
equaled 1.835 Å and 1.853 Å for the first molecule and second
molecule respectively. The corresponding O_H…O angles equaled 166.90° and 155.22°, while the
O…O distances are 2.640 Å and 2.620 Å for the first molecule and
second molecule respectively. The resulting structure may be presented as an
infinite chain.
|
|
|
|
Crystal structure of
compound 2 |
Conclusions
1. The novel stabilized derivatives of AsA
(1) were successfully synthesized with high yields and perfect purity.
2. Biological
studies showed that synthesized derivatives of AsA (1) active
participated in collagen synthesis of human foreskin fibroblasts.
3. These novel
stabilized derivatives of vitamin C can be used for production of various
cosmetic products.
Literature
1.
Ascorbic
acid: chemistry, metabolism, and uses. Advances in Chemistry Series, vol. 200,
Eds. Seib P.A., Tolbert B.M., Washington: American Chemistry Society, 1982.
2.
Davies M.B.,
Austin J., Partridge D.A., Vitamin C: its chemistry and biochemistry,
Cambridge: Royal Society of Chemistry, 1991.
3.
Farris P.K., Dermatologic
Surgery vol. 31, No. 7, 814-818, 2005.
4.
Segall A.I.,
Moyano M.A., Int. J. Cosmet. Sci. vol. 30, No. 6, 453-458, 2008.
5.
Hori Y.,
Akomoto R., Hori A., et al., J. Cosmet. Sci. vol. 60, No. 4, 415-422,
2009.
6.
Vitamin C
in health and disease,
Eds. Parker L., Fuchs J., New York: Marcell Dekker, Inc., 1997.
7. Palma S.,
Jimenez-Kairuz A., Fratoni L., et al., Farmaco, vol. 58, No. 12,
1271-1276, 2003.
8.
Duarte T.L.,
Cooke M.S., Jones G.D.D. Free Radic. Biol. Med , vol. 46, No. 1, 78-87,
2009.
9.
Takamizawa
S., Maehata Y., Imai K., et al., Cell Biol. Int., vol. 28, No. 4,
255-265, 2004.
10.
Tai A., Goto
S., Ishiguro Y., et al, Bioorg. Med. Chem. Lett., vol. 14, No. 3,
623-627, 2004.
11.
Shibayama H.,
Hisama M., Matsuda S., et al., Biol. Pharm. Bull., vol. 31, No. 4,
563-568, 2008.
12.
Nonius -2006.
COLLECT. Nonius BV, Delft, The Netherlands, 2006.
13.
Otwinowski
Z., Minor W., Methods in Enzymology, vol. 276, Macromolecular
Crystallography, Part A, Eds. Carter C.W., Sweet R.M., 307-326,
New York: Academic Press, 1997.
14.
Sheldrick
G.M., Acta Crystallogr., vol. A46, 467-472, 1990.
15.
Sheldrick
G.M., SHELXL97 and SHELXS97. University of Gottingen, Germany,
1997.
16.
Babtsov V.,
Shapiro Yu., Kvitnitsky E., Belakhov V., Patent USA 7 045 546, Chem.
Abst., vol. 138, 158807g, 2003.
17.
Cambridge
Structural Database, version 5.30, 2009.
18.
Bauerfeind
J.C., Int. J. Vitamin and Nutrition Res., suppl. Vol. 27 (Vitamins),
307-333, Gainesville, USA, 1985.
19.
Roig M.G.,
Rivera Z.S., Kennedy J.F., Int. J. Food Sci. Nutrition, vol. 44, No. 1,
59-72, 1993.
20.
Ìåëåíòüåâà
Ò.À., Òàáåð À.Ì., Õèì.-ôàðì. æóðí., ò. 29, ¹ 3, 60-65, 1995.
21. Yamamoto I., Fragrance
J. , vol. 32, No. 2, 61-73, 2004.
22.
Dalko M.,
Cavezza A., Patent France 2913686, Chem. Abst., vol. 149, 385781a, 2008.
23.
Shibayama H.,
Hisama M., Matsuda S., Ohtsuki M., Skin Pharmacol. Physiol. vol. 21, No. 4, 235-243, 2008.
24.
Higdon J.,
Frei B., In book: Cancer Chemoprevention, Eds. Kellof G.J., Hawk E.T.,
Sigman C.C., vol. 1, 485-510, 2004.
25.
Verrax J.,
BucCalderon P., Biochem. Pharmacol., vol. 76, No. 12, 1644-1652, 2008.
26.
Groeber U., Medizinische
Monatsschrift fuer Pharmazeuten, vol. 32, No. 7, 263-267, 2009.
27.
Frei B.,
Lawson S., Proc. Natl. Acad. Sci. USA, vol. 105, No. 32, 11037-11038,
2008.
28.
Gazivoda T.,
Wittine K., Lovric I., et al., Cabohydr. Res., vol. 341, No. 4, 433-442,
2006.
29.
Raic-Malic
S., Svedruzic D., Gazivoda T., et al., J. Med. Chem., vol. 43, No. 25,
4806-4811, 2000.
30. Gazivoda T., Plevnik M., Plavec J., et al.,
Bioorg. Med. Chem., vol. 13, No. 1, 131-139, 2005.
31.
Gazivoda T.,
Raic-Malic S., Marjanovic, et al., Bioorg. Med. Chem., vol. 15, No. 2,
749-758, 2007.
32.
Gazivoda T.,
Sokcevic M., Kralj M., et al., J. Med. Chem., vol. 50, No. 17,
4105-4112, 2007.
33.
Aguirre R.,
May J., Pharmacol. Therapeutic., vol. 119, No. 1, 96-103, 2008.