dr inż. Jerzy
Stanisław Kowalski
dr inż. Marek Mazur
dr hab. inż. Wojciech Wojciechowski
Institute of
Materials Engineering
Cracow University of Technology
Pad welding as a means of protection against corrosion
Abstract
The
article presents the possibilities that the arc pad welding creates in
protecting from corrosion various materials used in construction of equipment
operating in power industry. By application of pad welding, a coating (layer)
is deposited which not only protects
the material from corrosion caused by the flow of more or less aggressive media
(e.g. water, waste gases) but also prolongs the life of pad welded elements
since padding welds are usually characterised by high resistance to abrasive
wear. Pad welding is specially suitable for repair of parts damaged or
suffering standard wear and tear during operation in water installations, power
supply systems, etc.
INTRODUCTION
Failure
of pipelines handling industrial water to power plants or water from abyssal
strata is in the majority of cases caused, apart from the „human” factor, by
corrosion and erosion. This is due to the fact that water transported by
pipelines contains dissolved gases (including gases that speed up the corrosion
process), mineral compounds (e.g. calcium carbonate, chlorides of sodium,
calcium, or magnesium), and organic compounds. Additionally, water from the
subterranean springs often contains the acidic sodium carbonate, carbon
dioxide, sulphates and chlorides in concentrations much higher than the water in surface layers. Apart from the effect of
purely chemical nature, this also influences the pH value and the electrode
process that takes place at cathode spots with reactions of hydrogen or oxygen
depolarisation. The processes of erosion that take place on the internal
surfaces of steel pipes are accelerated by the water-carried particles of
undissolved quartz and argillaceous minerals which abrade the deposits growing
on the internal pipe walls. The next factor that makes the pipeline degradation
process more intense is the water flow rate. It is generally assumed that
increasing the rate of water flow usually speeds up the corrosion process,
caused by the corrosion products washed out in a strong jet of the flowing
liquid. In the latter two cases, the processes of corrosion and erosion are
observed to proceed at a rate definitely higher in these sections of the
pipeline where there are some elements of the technical infrastructure
restricting the free water flow, like reducing pipes, orifices and, particularly, elbows (bends).
An
important factor that affects the service life of pipelines is the temperature
of the handled water. This refers to both pipelines supplying water to power
plants and those which serve for drawing out and handling of abyssal and
geothermal waters. In the latter case, of particular importance is the
occurrence of temperature gradients that favour the formation of corrosion
cells.
The
above mentioned factors responsible for the corrosive and erosive degradation
of water pipelines mainly depend on the properties of the handled water, and
the tools that the technical supervising personnel have to control the
processes of corrosion are in this case very limited. The possibilities to
control these phenomena are definitely greater at the stage of designing and
selecting materials for the pipeline construction, specially as regards the use
of effective methods for intraoperational upgrading. Failure of pipeline is
always a serious problem in terms of both material and economy. The repair
requires large financial outlays for replacement of the damaged section and the
necessity of arresting the operation of the whole pipeline during the repair.
There are known various methods to reduce losses caused by the erosion of water pipelines. The choice of
the best method depends on detailed analysis of the pipeline operating
conditions. In most cases, the aim of all the methods applied currently is to
prolong the life of the structural elements of a pipeline using for their
construction clad plates, steel plates after thermal-chemical treatment, plates
surface etched with acids containing corrosion inhibitors, or plates coated or
painted with anticorrosive agents. The sections of the pipelines operating
under the most trying conditions of the corrosion-threatening environment are
made from the very expensive stainless steels, and in some, economically
justified, cases from the titanium-based alloys. Nevertheless, modern
technology has no efficient technical means to totally eliminate the corrosive wear
and tear of water pipelines. Therefore, the problem of current repair of these
elements is very important. Effective
and relatively cheap means of upgrading the pipeline sections damaged by
corrosion and erosion offers the technique of pad welding. The said technology
is very effective in making up for the losses of material and filling the
pittings in parent metal, due to the application of a layer of proper material
selected together with the welding electrode. Additionally, in the case of e.g.
plumbing fittings fabricated by the technique of powder metallurgy, pad welding
ensures the removal of surface porosities present in these products. Another
advantage of the pad welding process is the possibility to use this method in
repair of castings, specially at the places where, due to a long-lasting
operation, the sub-surface defects, like pores and voids, have been detected.
In the study presented here, a method of pad welding of the damaged surfaces of
the steel plates has been proposed as a
relatively simple and effective means for their upgrading, ensuring further
failure-free operation of pipeline.
All
over the world, corrosion is a serious problem. It destroys approximately 2 – 5 % of the gross national product, is
responsible for the contamination of natural environment and creates numerous
hazards to the human health and life. In 1989, in Poland, the losses due to corrosion and the related contamination
of natural environment reached 32% of overall material losses. The effects of
corrosion are present everywhere. Corrosion affects concrete, ferroconcrete,
harbour facilities, industrial devices, military infrastructure and military
equipment. As much as 80% of all financial means assigned for the repair of
bridges and viaducts are as a matter of fact assigned for combating the effects
of corrosion which attacks the industrial facilities and distribution systems.
CORROSION
Corrosion means all these
processes during which metal or alloy used as a structural material undergoes,
under the effect of environmental factors, a transformation from the metallic
state to a chemically bounded state.
Corrosive
reactions are not always harmful to the environment. The use of numerous
reactive metals (e.g. titanium, niobium, tantalum, and rare earth metals) is
possible in some environments due to the formation of a thin layer of corrosion
products that forms a barrier impeding further progress of reaction.
Depending
on the mechanism by which the corrosion process is proceeding, two basic types
of corrosion are distinguished, viz. the chemical and electrochemical
corrosion.
In aqueous solutions, the majority
of corrosion processes take place by electrochemical route, which means that
during reactions the electric charges are crossing the phase boundary. In terms
of structure, the surface of metal is not homogeneous but consists of minute
electrodes electrically shortcircuited by the metal. As long as the metal
remains dry, the current is not passing
and the corrosion does not take place. As soon, however, as it enters in
contact with water solution or with moisture contained in the humid atmosphere,
the local cells start acting and transform the metal into a product of
corrosion. The impurities present in metal enhance the corrosion, because
acting as microcells they exert a definitely stronger effect, and for this
reason, pure metals usually corrode much more slowly than metals with some
admixtures.
WELDING OF METALS vs CORROSION
The thermal processes that take
place during joining of metallic materials cause different phase
transformations which, to a smaller or greater extent, may occur within the
heat affected zone. This is specially true in the case of welding, partly also
in the case of pressure welding and soldering. The transformations change the
mechanical properties and corrosion resistance of thus produced joints.
WELDING
Depending on the welding technique,
the type of auxiliary materials and welding parameters, the welding process can
change in a different way the physico-chemical nature of metals and alloys. The
changes involve chemical reactions that proceed more quickly at higher
temperatures and precipitation of compounds, like carbides, oxides, nitrides,
sulphides, phosphorides, and intermetallic compounds. Moreover, during the
process of welding, there is a recrystallisation of metal, the growth and
deformation of crystal grains, sometimes the formation of new structural phases
which move and agglomerate, giving rise to lattice defects and internal
stresses of uni- or biaxial character, caused mainly by the shrinking power of
the welded metal.
There
is always some degree of inhomogeneity between the welded joint and parent
metal. Its symptoms are differences in structure, mechanical properties and the
state of stress inside metal. The inhomogeneity may enhance the corrosion rate,
specially as regards the electrochemical and stress-induced corrosion. To
eliminate or reduce this inhomogeneity, additional thermal or mechanical treatments
are applied.
THE CORROSION OF WELDED STEEL JOINTS
In the majority of cases, the
welded joints are less resistant to corrosion than the parent metal. Normally,
all welded structures made from carbon and low-alloy steels are protected from
the atmospheric corrosion with special coatings applied by painting. Quite
often, however, it happens so that on the welded joints the varnish is spalling
and falling off, exposing the bare metal surface, which is now running the
greater risk of corrosion. To avoid spalling of paints and enamels, the welded
joints and their neighbourhood should be cleaned mechanically before painting
with, e.g. hot water, and dried.
The laboratory tests of the
corrosion resistance of the joints welded from carbon and low-alloy steels are
carried out in accordance with PN-76/H-04601 to 04604.
In welded structures operating in the
aqueous media containing, e.g., alkaline solutions, soon after the installation
has been introduced to use, the cracks of orientation transverse to the weld
appear and are penetrating to the parent metal. It has been observed that
corrosion of this type may take place only under the combined effect of
mechanical stresses and chemical reaction of the hydroxide solution. Therefore,
to arrest the development of corrosion, the welded joint should be
stress-released by annealing at a temperature of 650oC, or possibly
by subjecting it to the thermal and mechanical treatment. The water preheaters
in modern power boilers and pipe systems through which the aggressive media are
flowing are composed of the pipe sections joined together by welding. Often in
these joints some perforations appear due to the selective corrosion which
usually occurs near the weld end.
To avoid corrosion of this type, it
is recommended to make the girth weld with several runs instead of one single
run, laying the runs in such a way as to make the beginning of the new run and
the end of the previous one shift in respect of each other.
The more numeous are the runs, the
thinner is the electrode, and the lower is the welding current, the more
reliable is the joint. The welded joints in pipes expected to operate under the
conditions of pressure should be, in principle, subjected to heat treatment.
For the construction of some pipelines, specially those
handling potable water and water used by food industry, chromium steels of the
0H13, 0H13J, 0H17 and 0H17T grades are commonly used nowadays, though the austenitic
0H18N9 and 1H18N9 grades are also observed to gain popularity. These steels are
usually welded with electrodes giving the weld metal of an austenitic structure
in grades E 13 B 22, E 17 B 22, E 19 9
R 22 or E 19 9 B 22 (acc. to EN 1600). In some environments, the welded joints
of these steels are susceptible to intercrystalline and stress corrosion.
If the welded joints in the above
mentioned steels are expected to offer some resistance to the intercrystalline
corrosion, they should be subjected to heat treatment which consists in
annealing at a temperature of 800oC for 2 hours (when the weld has
been made with austenitic electrodes), or 4 hours (when a ferritic filler has
been used), followed by slow cooling together with furnace to a temperature of
600oC and rapid cooling in the air, next. The necessity to use so
complex heat treatment seriously limits the range of application of these
steels in construction of chemical apparatus, specially because of the grain
growth, and hence higher brittleness of the material.
The
risk of occurrence of the intercrystalline corrosion in a welded joint depends
on the carbon content in welded steel and in metal deposited from the electrode
used for welding; it also depends on the heat volume supplied in a time unit.
To produce welded joints resistant to the intercrystalline corrosion it is
recommended to use the steel of low carbon content or steel stabilised with
titanium. If the joint should be deposited with several runs, the layer of the
weld contacting directly the aggressive medium should be made with an electrode
of low carbon content (0,02...0,03%C), or it should be stabilised with, e.g.,
niobium.
The knife-line corrosion attack is
of an intercrystalline nature and it occurs specially in welded joints made
from the chromium-nickel-molybdenum steels. Corrosion of this type occurs
mainly at the welded joint-parent metal interface, and it may destroy in a
relatively short time the whole joint. As preventive means it is recommended to
use electrodes of low carbon content, possibly electrodes that will give the
weld metal of a ferritic-austenitic type, or to apply a stabilising heat
treatment..
The
welded joints made in chromium-nickel and chromium-nickel-molybdenum steels
with the commonly used electrodes are less resistant to stress corrosion than
the parent metal. In some cases, the resistance of the welded joint to the
stress corrosion is only 10% of the parent metal resistance.
PAD
WELDING
Pad
welding is the process of application of metallic coatings where a characteristic
feature is remelting of the substrate
material on which the padding weld has
been deposited.
When for pad welding the material of
properties different than the properties of parent metal is used, there are
some side effects resulting from mixing of the base (pad welded) metal with the
fused padding metal. The degree to which these two materials get mixed together
is the greater, the greater is the depth of penetration of the padding weld
into the base metal.
The
content of carbon in the individual layers of a padding weld depends on the
content of carbon in parent metal and in the filler, and also on the
penetration depth. Therefore, to obtain the highest possible corrosion
resistance, both parent metal and filler metal should have the lowest possible
content of carbon with the highest possible content of elements, like chromium
and nickel.
Among
the numerous methods available, the smallest penetration depth into the pad
welded metal gives the process of pad welding with shielded arc and strip
electrodes, the highest – the pad welding in protective gas atmosphere with
consumable electrodes, and with shielded arc and one wire electrode.
Pad welding with strip electrodes is
widely used by industry in the manufacture of
heavy-wall tanks (e.g. for the nuclear power industry), which should
have a very high corrosion resistance in their inside part.
METHODS
TO TEST THE CORROSION
The practical aim of corrosion testing
is to determine the life of metals or alloys during performance under given
conditions. The results of these tests should give an answer to the question in
what way the metals or alloys will behave on performance.
At present, the following tests are
conducted:
1. Laboratory tests
under the conditions created artificially by means of various devices.
2. Field tests under
actual operating conditions.
3. Laboratory tests
carried out on real facilities and under the real operating conditions.
Numerous methods of corrosion
measurement are available, but they all can be divided in qualitative and quantitative methods.
QUALITATIVE
METHODS consist in observations of the object exposed
to corrosion and are divided into:
a) direct (visual
observations with unarmed eye)
b) macroscopic
(observations under magnifying glass)
c) microscopic
(observations under microscope)
d)
evaluation of changes in
mechanical properties
All these methods are characterised
by great simplicity. The last method is very easy and handy in determination of
the internal (hidden) corrosion.
QUANTITATIVE
METHODS
These
methods consist in the determination of:
a) the time when on
a given surface the first corrosion spots appear and the corrosion spots count
in a time unit;
b) the change in
specimen thickness and depth of pits, or loss of weight;
c) increase of
weight when the corrosion products are insoluble and stick to the specimen. If
this is the case, the corrosion
is expressed as an increment in
weight in a time unit, e.g. g/m2·year.
d) mechanical
properties during the tensile test (Rm and A), or technological
properties during the bending test (the number of flexions). In this case the corrosion
is expressed as a percent drop in the value of the tested property during one
year.
Besides the tests mentioned above,
there are also numerous other, often very complicated, tests, like the
determination of the volume of the soluble corrosion products in a solution, of
the volume of evolved hydrogen or absorbed oxygen, changes in electric
resistance, changes in the thermal effect, changes in the ability to reflect
light, etc.
OWN INVESTIGATIONS
TESTING OF THE
PADDING WELDS CORROSION RESISTANCE
Testing
of corrosion resistance was carried out on the steel specimens with and (for
the sake of comparison) without padding welds.
The aim
of the investigations was to evaluate the behaviour of pad welded elements (the
pad welded specimens) under the performance conditions. To achieve this goal,
simulation tests were carried out under the laboratory conditions.
Two methods of the evaluation were
applied:
1) visual – the
evaluation of changes in the appearance of the specimen surface,
2) evaluation of
changes in the specimen weight and dimensions.
Evaluating
the surface condition, it is necessary to evaluate the type and colour of the
corrosion products, the severity of corrosive attack, and the symptoms of
corrosion. The qualitative evaluation should indicate the type of corrosion,
its character and if there are any local damages caused by the corrosion or
not.
TEST
MateriaL FOR INVESTIGATION OF THE CORROSION RESISTANCE OF PADDING WELDS
Corrosive
environment: water; aqueous solutions of H2SO4, HNO3,
HCL,
NaCl, simulating
the saturation of water from the abyssal or geothermal springs with the above
mentioned compounds.
Basing on the reference literature and
the results of own investigations it has been assumed that the 5% concentration
of corrosive media is corresponding with great probability to the real
conditions of operation of a steel (metal) structure, e.g. of a pipeline for
handling of the abyssal and geothermal waters, taking into account the factors,
like water temperature, flow rate, gas saturation ratio, and erosive wear
during an uninterrupted operation for 25 years.
Table 1. Chemical composition of steel S235JR
(St3S);
in accordance with a
standard EN 10025: 1993
C
|
Mn
|
Si
|
P |
S |
Cr |
Ni |
|
0÷0,22 |
0÷1,1 |
0,1÷0,35 |
0÷0,05 |
0÷0,05 |
0÷0,3 |
0÷0,3 |
Table 2. Chemical composition of weld metal [%]
|
Designation |
Type |
C |
Si |
Mn |
Cr |
Ni |
Mo |
|
1 |
E19 9 B 22 |
0,07 |
0,3 |
1,2 |
19,5 |
9,0 |
|
|
2 |
OK16.31 |
0,03 |
0,6 |
1,1 |
19,0 |
12,0 |
2,5 |
|
3 |
OK 61.30 |
0,03 |
0,8 |
0,6 |
19,0 |
10,0 |
0,75 |
|
4 |
OK 12.51 |
0,1 |
0,9 |
1,5 |
|
|
|
|
0 |
No weld |
||||||
Table 3. Corroding medium.
|
Designation |
5%
of water solution |
|
S |
H2SO4 |
|
N |
HNO3 |
|
H |
HCl |
|
C |
NaCl |
TEST METHODS
Tests and investigations were carried
out on the specimens of S235RJ steel. On some of the specimens, the padding
welds were deposited with wire electrodes and with coated electrodes, following
guidelines adopted in the research program. The padding welds were deposited
semi-automatically under gas shielding and manually with coated electrodes (MAG
proccess).
PARAMETERS
OF THE PAD WELDING PROCESS
Pad welding under gas shielding:
Arc voltage - U1=18,3 [V], Welding current - I1=128 – 132 [A]
Wire feed rate - ve=3,45
[m/min], Protective gases - CO2, Argon
Manual pad welding with coated electrodes:
Electrode diameter
- 4,0 [mm], Welding current: 114 [A]
PROGRAM OF RESEARCH
On S235JR steel plates, altogether 16
padding welds were deposited – four with each type of the electrode and wire,
and then the pad welded specimens and the four specimens without padding welds
were subjected to the attack of aqueous solutions of H2SO4,
HNO3, HCL and NaCl.
Table 3. Project of tests in accordance with the table 2 - 3
|
Weld metal |
Corroding
medium |
|||
|
H2SO4 |
HNO3 |
HCL |
NaCl |
|
|
E 19 9 B 22 |
1S |
1N |
1H |
1C |
|
OK. 16.31 |
2S |
2N |
2H |
2C |
|
OK 61.30 |
3S |
3N |
3H |
3C |
|
OK 12.51 |
4S |
4N |
4H |
4C |
|
No weld |
5S |
5N |
5H |
5C |
(Detali of the diagram above)
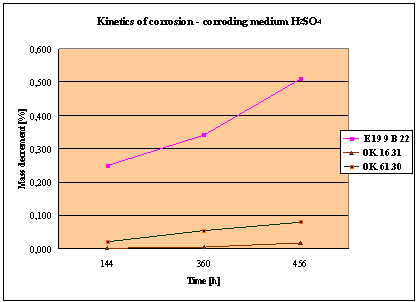
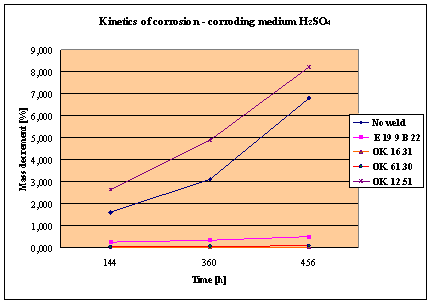
Fig. 1. Mass decrement of surfaced plate specimen; water solution H2SO4
(Detali of the diagram above)
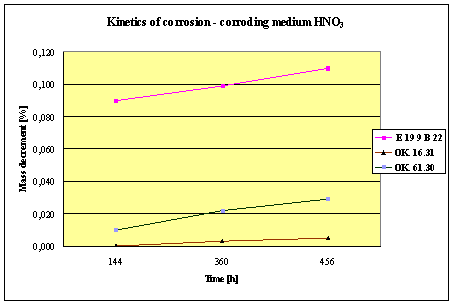
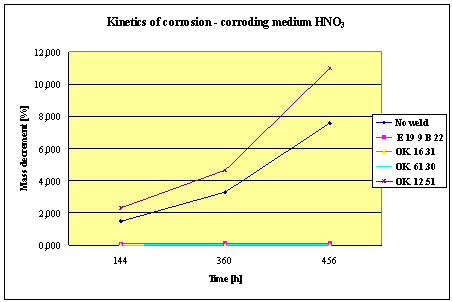
Fig.2. Mass decrement of surfaced plate specimen; water solution HNO3
(Detali of the diagram above)
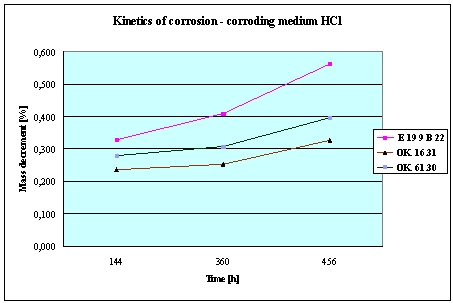
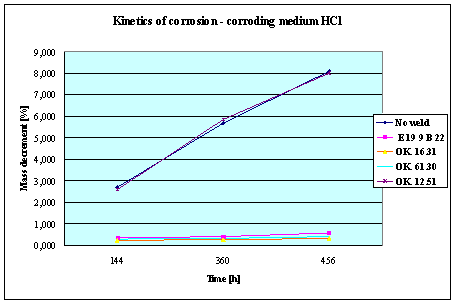
Fig.3. Mass decrement of surfaced plate specimen;
water solution HCl
(Detali of the diagram above)
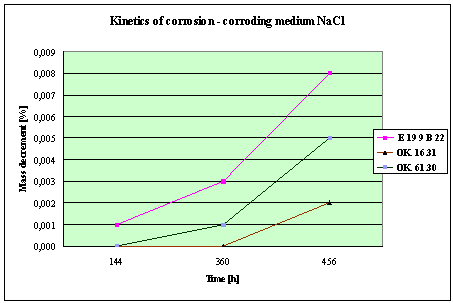
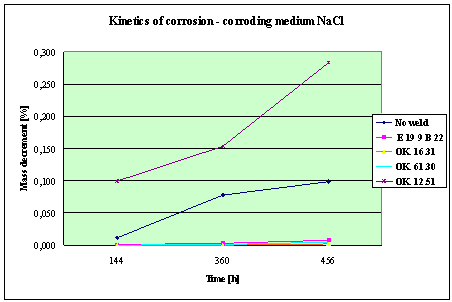
Fig. 4. Mass decrement of surfaced plate specimen;
water solution NaCl
THE RESULTS AND CONCLUSIONS
1. The corrosion
resistance of the steel and padding welds depends on the type of the corrosive medium (the contamined water).
2. The pad welding
with wire electrodes, which give the weld metal of the same properties as the
common steel, makes no sense. As results from the plotted graphs, the corrosion
resistance of such padding welds is lower than that of the steel itself.
3. The highest
corrosion resistance had the specimens with padding welds deposited with an
Autrod OK. 16.31 wire electrode containing 2,5 % Mo. The results almost
identical were obtained when electrodes giving the weld metal of much lower Mo
content were used, which is quite important from the economic point of view.
4. Slightly inferior results
were obtained in the case of padding welds deposited with E 19 9 B 22 (ES 18-8
B) electrodes. The drop of corrosion resistance is due to the effects that
accompany the reactions taking place between the components of the electrode coating (specially if they are
of a basic reaction) and parent metal. An important factor improving this
resistance is without any doubt the exact removal of slag originating from the
melted electrode coating and periodical (according to the technical
possibilities) cleaning of the padding welds or welded joints.
5. The greater losses
– independent of the type of the padding weld – occurred when water was
acidified with sulphuric acid and with hydrochloric acid, which is probably due
to depolarisation and its type related to either oxygen or hydrogen.
6. It seems advisable
to use the technology of pad welding in structures and facilities that should
offer high corrosion resistance, specially as regards current repairs and
emergency repairs (the latter ones being of major importance), instead of
replacing the whole sub-assemblies. Pad welding is fully justified from the
point of view of economy. It provides, moreover, some opportunities to reduce
the volume of the accumulating waste parts.