Increasing
power conversion efficiency of DSSC based on dioxide titanium nanorods
Serikov T.M.,
Ibrayev N.Kh., Amanzholova G.S.
Institute of
molecular nanophotonics, Buketov Karaganda State University
e-mail:serikov-timur@mail.ru
Republic of Kazakhstan
Abstract
Membranes of nanorods
of titanium dioxide are synthesized in the work by hydrothermal method. On a
surface of nanorods nanoparticles of titanium dioxide have been settled. By the
nitrogen adsorption method (BET method) sizes of specific surface have been
measured, full pore volume, distribution of pore by the sizes in the membranes
formed by nanorods (NRs) and nanorods with the spherical nanoparticles of
titanium dioxide (NRs+NPs) settled on a surface. By the method of scanning
electronic microscopy morphology of a surface of the synthesized membranes has
been studied. On a basis of the synthesized membranes solar cells have been
collected by sensibilized dye. Volt-ampere characteristics and efficiency of
cells are measured.
Key words: nanorods
and nanoparticles of titanium dioxide, specific surface area, efficiency, DSSC
Introduction
The solar power is
one of the most actively developing branches of power industry now. Energy of
the sun is available to everybody, is free, almost inexhaustible, and process
of its transformation to electric energy doesn't exert negative impact on
environment. However, today the solar power, mainly on the basis of silicon,
occupies less than 1% in universal balance of the made electric power. It is
connected with difficult manufacturing techniques and high cost of silicon
solar elements that interferes their wide use [1].
The new generation
of solar elements on the basis of oxidic semiconductors (TiO2, ZnO,
SnO) and organic materials (DSSC) has prospect of reduction in cost of the
developed electric power and simplification of production. Use in sensibilized
dyes solar cells of nanostructures on the basis of titanium dioxide caused a
huge interest of researchers after O’Rigan and Grettsel's well-known work was published
in 1991 [2]. From publications it is known that morphology, structure and
design of electrodes play an important role in absorption of photons,
electronic transport and efficiency [3,4].
For receiving
nanostructures on the basis of dioxide such methods as hydrothermal [5],
zol-gel [6], methods of electrochemical anodizing [7] are used as a result of
which structures of various morphology, such as nanoparticles, nanotubes,
nanowires and nanorods are received. Use of nanoparticles of titanium dioxide
is limited because of 3-dimensional electronic transport as connection between
nanoparticles influences a possibility of transition of an electron from nanoparticle
to nanoparticle will lead to decrease of efficiency of transport of an
electron. Perspective materials for solar cells are structures with
one-dimensional transport of electrons to which it is possible to carry
nanotubes, nanorods and nanothreads of titanium dioxide. When using such
structures one-dimensional transport of electrons along walls will be observed.
It will reduce time of transfer of electrons from the centers of generation of
a charge to electrodes, and also, at the correct construction of electrodes,
there will be a smaller amount of the defects interfering transport of
electrons [8,9,10]. Nanorods of titanium dioxide are one of perspective
materials with one-dimensional transport of electrons. One of their main
advantages is the possibility of synthesis of nanorods on glass with a
conductor layer FTO that will allow to reduce losses on semiconductor/FTO
border. Besides, nanorods possess the developed surface.
It is known that
the amount of the absorbed light a photovoltaic cell directly depends on number
of molecules of the dye adsorbed by a semiconductor surface. Increase in a
specific surface of a semiconductor oxidic layer will allow to adsorb bigger
quantity of molecules of dye and it will lead to growth of absorption of a
sunlight and increase in concentration of charge carriers in a semiconductor
layer.
Problem of this
work is the increase in a specific surface of the membranes formed by nanorods of
titanium dioxide due to modification of their surface by TiO2 nanoparticles.
Experimental part
Rutile-anatase
nanostructures of TiO2 have been
prepared on FTO glasses (TES-8, 8 Ω/ sq.m) by means of two consecutive stages of hydrothermal synthesis. FTO
of glasses were washed out by processing of ultrasound in solution of the
deionized water, acetone and a 2-propanol (a volume ratio 1: 1: 1) within 30
minutes, then they were placed in a stainless steel vessel with fluoroplastic
coating of 50 ml. FTO glass was established in a vessel with the carrying-out
party down, in the solution containing 15 ml of the deionized water, 15 ml of
hydrochloric acid (36.5-38.0%, Sigma-Aldrich), and 0,5 ml of titanium butoxide (titanium
butoxide, 97%, Sigma-Aldrich). The stainless steel vessel then is closed and
placed in the convective furnace at 1400 C
for 20 hours. According to the procedure described above vertical nanorods of TiO2 were present on FTO glass. The
second stage is as follows: nanorods on FTO glasses placed in the solution
containing 35 ml of H2O, 2,5 ml of
sulfuric acid (36.5-38.0%, Sigma-Aldrich), and 1 ml titanium butoxide (titanium
butoxide, 97%, Sigma-Aldrich) in the same stainless steel vessel with a teflon
covering. The steel vessel was placed in the convective furnace at 1800 C and maintained at this temperature
during 6 hours. The received sample was washed out and dried. The sample then
was calcinated at 400 î C during 2 hours.
The rutile TiO2 nanorods received at the first stage
of hydrothermal synthesis subjected to water processing of TiCl4 (0,4 M TiCl4
during 1 hour at ambient temperature) [11,12].
The morphology of a
surface and cross cut of samples have been received on the scanning electronic
microscope MIRA 3LMU (Tescan, the Czech Republic).
Measurement of a
specific surface was taken by BET method, distribution of the size of pores,
dependence of pores volume on their diameter have been received from isotherms
of adsorption and a desorption of nitrogen on the measuring complex Sorbi-MS
(Russia). Thermal training of samples was carried out at a temperature of 1000Ñ within 180 minutes in the block of prepreparation
"SorbiPrep".
In organic solar
cells dye is one of key components. Efficiency of cells depends on its
absorptive capacity, concentration. We used N719 (Sigma-Aldrich) dye which is
characterized by a high absorptive capacity and is often used in the photovoltaic
cells. Sorption of dye was carried out from ethanolic solution with
concentration 10-4 mol/l during 18 hours. As electrolyte in a cell
Iodolyte H30 was used (Solaronix, Switzerland). As laying between a working
electrode and an electrode of yield in a solar cell served the membrane, in
thickness of 25 microns the Meltonix brand (Solaronix, Switzerland).
Volt-ampere
characteristics were measured in the standardized conditions at radiation by
light of a source with the range imitating solar (Air Mass (AM) 1,5). Standard
power of a source made 100 mW/cm2 (PET PHOTO Emission TECH., INC.).
EIS measurements
were taken under the standard feigned sunlight of AM 1.5, 100 of mV/cm2 (PET
PHOTO Emission TECH., INC.) on an impedancemetre Z–500PRO (Elins), amplitude
and range of frequency of the enclosed sinusoidal signal – 15 mV and 500
kHz-100 kHz respectively.
Results and their discussion
Morphology of the membrane
surface from nanorods of titanium dioxide received on the scanning electronic
microscope is presented in figure 1.
|
|
|
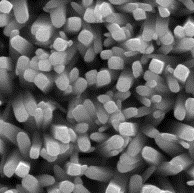
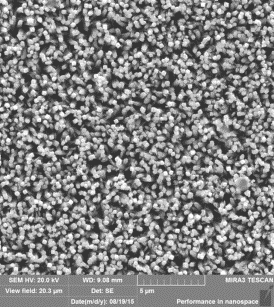
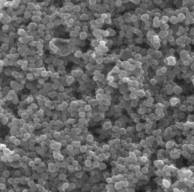
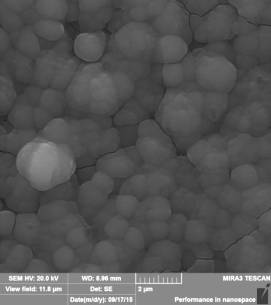
Fig. 1 - a) nanorods
of titanium dioxide after the first stage; b) nanorods of titanium dioxide on
surface of which nanoparticles of titanium dioxide at the second stage are settled
In a picture it is
visible (figure 1, a) that on a surface of FTO glass the nanorods of titanium dioxide
located perpendicularly to the substrate plane are formed. Average diameter of nanorods
after the first stage of synthesis makes 100-120 nanometers, and length is 3,5
microns. At the second stage on a surface of the nanorods received at the first
stage spherical nanoparticles TiO2,
with an average diameter of 200-250 nanometers have been settled (figure 1, b).
Total thickness of membrane has made 4 microns.
For measurement of
a specific surface, the membranes have been separated from glass and are placed
in an adsorber. Measurement was taken at a temperature of liquid nitrogen. For
release of a surface from moisture samples during 180 minutes were calcinated
and blown at a temperature of 100 0Ñ. Results of measurements are given in table 1.
Table 1 Specific
surface of samples after the first and second stage
|
¹ |
Specific
surface area SBET,
ì2/g |
Full pore
volume Vp, ñì3/g |
|
TiO2 NRs |
29 |
0,042 |
|
TiO2 NRs+NPs |
38 |
0,061 |
As it is seen from
table 1, application of nanoparticles of titanium dioxide on a surface of nanorods
leads to increase of specific surface and total pore volume. Small increase of Sóä can be explained with the fact that nanoparticles
possess more developed surface, than nanorods.
Curves
of volt-ampere characteristics are given in figure 2.
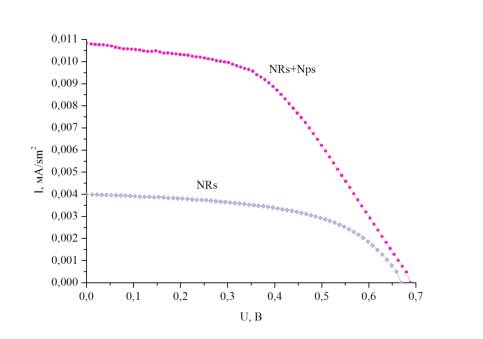
Figure 2 -
Volt-ampere characteristics of solar cells
As it is seen from
the figure, at addition of nanoparticles in structure of nanorods of titanium dioxide
current density of short circuit has increased by 2,5 times. DSSC on the basis
of the massif of nanorods and nanoparticles has shown the greatest efficiency
of transformation of solar energy to electric. The more the specific surface
area, the more amount of dye can be on the single area of a substrate.
Therefore, bigger quantity of electrons are injected under the influence of
sunlight that increases cell current. The main indicators of solar cells
received as a result of measurement VAC are given in table 2.
Table 2 -
Photo-electric characteristics of solar cells
|
¹ |
Specific surface area, SBET,
m2/g |
Open
circuit voltage, Voc, |
Short-circuit current Jsc (mA/cm2) |
Fill factor, FF |
Rs,
Om |
Rsh,
Om |
Efficiency η, % |
|
NRs |
29 |
0,66 |
0,004 |
0,54 |
265 |
3875 |
1,43 |
|
NRs+NPs |
38 |
0,68 |
0,01 |
0,47 |
122 |
2839 |
3,56 |
From
tabular data it is seen that in the result of modification of a surface of nanorods
resistance is decreased by the nanoparticles of titanium dioxide Rs.
For a real solar element resistance RS consists of consistently
included resistance which are responsible for quality of contact layers, and Rsh
resistance (leak resistance which in ideal SE is supposed to be infinitely big)
reflects possible channels of current leakage. That is, in the ideal solar
element Rs→0, à Rsh→∞.
The
analysis of data of table 2 shows that at modification of a surface of nanorods
spherical nanoparticles along with current growth of short circuit the size Rs
falls that demonstrates improvement of ohmic contact in membrane. At the same
time modification of a surface of nanorods - to falling of size of the shunting
resistance. In general, the efficiency of solar cells on the basis of “nanorods-nanoparticles”
system TiO2 increases in comparison with cells on the basis of nanorods.
One of
the most widely used methods at research of electrophysical characteristics of
solar cells is measurement of an electric impedance [13]. The essence of the
method consists in influence by a signal in the form of sinusoidal wave and
supervision over behavior of system in response to this indignation.
Impedansogram of cells on the basis of porous membranes TiO2,
received at different stages of synthesis, are given in figure 5.
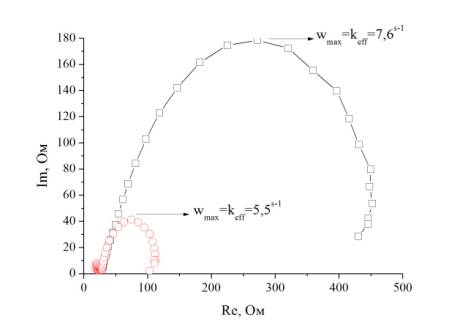
Fig. 5 – Impedansogram of a cell
On the basis of the
received ranges of an impedance and the technique described in work [14] for
cells the effective coefficient of diffusion of electrons Deff,
effective speed of recombination keff,
effective time of life of an electron τeff, resistance of
electronic transport in membrane of titanium dioxide Rw, resistance of transfer of a charge connected with
recombination of an electron Rk, Con defined as Con=RkLkeff have been calculated. The
received results are given in table 3.
Table 3 Electrophysical characteristics of the cells received at
different stages of synthesis
|
Sample |
Deff (cm2 s-1) |
keff (s-1) |
τeff (s) |
Rk (Om) |
Rw (Om) |
Con (Om cm s-1) |
L, mkm |
|
TiO2 NRs |
12*10-5 |
7.6 |
0.13 |
413 |
13.7 |
1.09 |
3.5 |
|
TiO2 NRs+NPs |
26*10-6 |
5.5 |
0.18 |
92 |
3,06 |
0.2 |
4 |
Speed of
recombination keff is
determined by peak frequency ωmax of the central
arch (in the range 500 kHz-100 MHz) ![]() , Rk, is determined
by diameter of the central arch, Rk/Rw is defined from a
form of the central arch. When the arch is the correct circle, Rk is
much more than Rw. The effective coefficient of electrons diffusion Deff is defined as
follows:
, Rk, is determined
by diameter of the central arch, Rk/Rw is defined from a
form of the central arch. When the arch is the correct circle, Rk is
much more than Rw. The effective coefficient of electrons diffusion Deff is defined as
follows: ![]()
From table 3 it is seen
that the cell on the basis of nanorods and nanoparticles of titanium dioxide
received after the second stage of synthesis possesses the best
electrotransport properties. The minimum value of resistance of electronic
transport in TiO2, the low speed of
recombination and longer effective time of life of an electron is observed for
it.
Conclusions
Thus, in the result
of hydrothermal synthesis the massifs of nanorods of titanium dioxide sent
perpendicularly to the substrate plane are synthesized. On the received nanorods
spherical nanoparticles of titanium dioxide have been settled. Sizes of a
specific surface and pore volume of membranes have been compared. It is
established that at settlement of nanoparticles of titanium dioxide on the
surface of nanorods TiO2 the
specific surface and pore volume increases. By measurements of volt-ampere
characteristics it is established that current density grows, the factor of
filling and efficiency of solar cells increases. At modification of a surface
of nanorods TiO2 nanoparticles Rs
resistance, responsible for quality of interlaminar contacts decreases. At that
time channels of current leakage increase. As a result of measurement of an
impedance of a range it is established that the cell on the basis of nanorods and
nanoparticles of titanium dioxide received at the second stage of synthesis
possesses the best electrotransport properties.
References:
[1] Saenko A.V.
Development and research of sensibilized by the dye solar elements on the basis
of titanium dioxide: Abstract … dissertation on competition of Science degree
of Cand.Sc. - Taganrog. 2013. – p.4-5.
[2] O'Regan B.,
Gratzel M. A low-cost, high-efficiency solar cell based on
dyesensitizedcolloidal TiO2 films // Nature. – 1991. - Vol. 353. – P. 737–740.
[3] Yu J.G., Fan J.J.,
Zhao L. Dye-sensitized solar cells based on hollow anatase TiO2
spheres prepared by self-transformation method // Electrochim Acta. – 2010.
- Vol. 58. – Ð. 1501-1507.
[4] Cauda V., Pugliese D., Garino N., Sacco A., Bianco
S., Bella F., et al. Multi-functional energy conversion and storage
electrodes using flower-like zinc oxide nanostructures // Energy. – 2014. -
Vol. 353. – Ð. 639-646.
[5] Yang J., Mei
S., Ferreira J.M.F. Hydrothermal synthesis of TiO2 nanopowders from
tetraalkylammonium hydroxide peptized sols // Materials Science and
Engineering: C. - 2001. - Vol. 15. - ¹1-2. - P. 183-185.
[6] Sahil Sahni, S.
Bhaskar Reddy, B.S. Murty Influence of process parameters on the synthesis of
nano-titania by sol–gel route // Materials Science and Engineering: A. - 2007.
– Vol. 452. - P. 758–762.
[7] Gong D., Grimes
C.A., Varghese O.K., Hu W., Singh R.S., Chen Z., Dickey E.C. Titanium oxide
nanotube arrays prepared by anodic oxidation // Journal of Materials Research.
- 2001. – Vol. 16. – P. 3331–3334.
[8] Zhao L., Yu J.G.,
Fan J.J., Zhai P.C., Wang S.M. Dye-sensitized solar cells based on ordered
titanate nanotube films fabricated by electrophoretic deposition method //
Electrochem Commun. – 2009. - Vol. 11. – P. 205-215.
[9] Jiu J.T., Isoda
S., Wang F.M., Adachi M. Dye-sensitized solar cells based on a
single-crystalline TiO2 nanorod film // J Phys Chem B. - 2006. - Vol. 110. – Ð. 208-292.
[10] Fujihara K.,
Kumar A., Jose R., Ramakrishna S., Uchida S. Spray deposition of electrospun
TiO2 nanorods for dye-sensitized solar cell // Nanotechnology. –
2007. – Vol. 18 – P. 37-41.
[11] Feng X.,
Shankar K., Varghese O.K., Paulose M., Latempa T.J., Grimes C.A., Vertically
aligned single crystal TiO2 nanowire arrays grown directly on
transparentconducting oxide coated glass: synthesis details and applications //
Nano Lett. 2008. – Vol. 8 – P. 3781–3786.
[12] Baxter J.BWalker., A.M., Ommering K.V., Aydil E.S., Synthesis and
characterization of ZnO nanowires and their integration into ye-sensitized
solar cells // Nanotechnology. – 2006. – Vol. 17 – P. 304-312.
[13] Bisquert, J. and F. Fabregat–Santiago,
Impedance Spectroscopy: A General Introduction and Application to
Dye–Sensitized Solar Cells, in Dye–Sensitized Solar Cells, K. Kalyanasundaram,
Editor. CRC Press. - 2010. – P. 457–609.
[14] Motonari A.
M., Sakamoto M., Jiu J., Ogata Y., Isoda S. Determination
of Parameters of Electron Transport in Dye-Sensitized Solar Cells Using
Electrochemical Impedance Spectroscopy. - J. Phys. Chem. B. - 2006. V.110. - P.13872-13880.