Áèîëîãè÷åñêèå íàóêè/ Ñòðóêòóðíàÿ áîòàíèêà è áèîõèìèÿ
ðàñòåíèé
Zhumabayeva S.1, Gibadilova A. 2,
Poplavskiy N.1
1Kokshetau State University; 2Kokshetau Medical College,
Kazakhstan
Study of rapeseed oil by NMR-spectroscopy method
Vegetable oils are important due to their nutritional value mainly. They
are mandatory components of food and a source of energy and plastic material
for humans. Biological properties of oils are determined by the fatty acid and
triglyceride compositions, as well as by the presence of biologically active
compounds (sterols, tocopherols, phospholipids, carotenoids, sterols, etc.).
In triglycerides OH-group of glycerol is bound to the COOH-group of
fatty acid to form an ester bond. Fatty acids differ in molecular weight and
degree of saturation. In the triglycerides they can be attached to glycerin in
three different positions which defines a large variety of optical isomers and
spatial triglycerides. Therefore the investigation of vegetable oils as a
mixture of triglycerides is a difficult task [1].
To determine the fatty acid composition in triglycerides of vegetable
oils classical methods of analysis such as chromatography and mass spectroscopy
are applying. In the last decade, research is based on nuclear magnetic
resonance (NMR) spectroscopy. This method is non-destructive and doesn’t require
multi-step analysis which is convenient for the screening of large quantities
of oil samples [2-4].
Recently, interest to rapeseed
oil has grown, which, in addition to food purposes, is promising as a source of
industrial oil, high protein animal feed and as a raw material for biodiesel
production.
The aim of our research is comparative study of rapeseed oil by NMR
spectroscopy method.
Methodology
Rapeseed oil was produced from seeds of 2012 years’ harvest. The extraction of rapeseed oil was performed
with diethyl ether in a Soxhlet apparatus.
To perform the 1H NMR analysis oil samples (0.5 ml) were
dissolved in 0.5 ml of deuterated chloroform (CDCl3). Records of spectra
were done on the NMR spectrometer JNM-ECA 400 of Jeol company (Japan) with an
operating frequency of 400 MHz. Chemical shifts are expressed in ppm.
Results and discussion
1H NMR spectra of rapeseed oil we studied is shown in fig.
1. It is known that the main constituent of vegetable oils are glycerol esters
of various saturated (palmitic, stearic) and unsaturated (oleic, lenoleic,
lenolenic) fatty acids [1, 5-7].
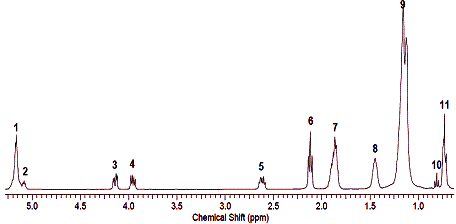
Fig. 1. 1H
NMR spectra of rapeseed (B) oil. 400
MHz (solvent: CDCl3).
Signal
1 indicates the presence of olefinic
protons. In the current signal (5.15-5.20 ppm) we observe a broad multiplet,
indicating the vinyl protons (-CH=CH-) of the double bonds in the chains of the
all unsaturated fatty acids [6].
Table 1. Signals of
1H NMR spectra and the functional groups of rapeseed oil’s
components
|
Signal |
Functional groups |
The multiplicity * |
δ, ppm |
|
1 |
-ÑÍ=ÑÍ- |
t |
5.12-5.20 |
|
2 |
-ÑÍ-Î-ÑÎR |
t |
5.05-5.10 |
|
3 |
-CH2–O–CO–C
|
dd |
4.10-4.16 |
|
4 |
-CH2–O–CO–C
|
m |
3.92-4.03 |
|
5 |
-ÑÍ=ÑÍ-ÑÍ2-ÑÍ=ÑÍ- |
t |
2.57-2.66
(m) |
|
6 |
-ÑÍ2-ÑÎÎÍ |
t |
2.08-2.15 |
|
7 |
-ÑÍ2-ÑÍ=ÑÍ- |
m |
1.82-1.93 |
|
8 |
-ÑÍ2-ÑÍ2-ÑÎÎÍ |
m |
1.40-1.49 |
|
9 |
-(ÑÍ2)n- |
d |
1.09-1.20 |
|
10 |
-CÍ=ÑÍ-ÑÍ2-ÑÍ3 |
t |
0.78-0.84 |
|
11 |
-ÑÍ2-ÑÍ2-ÑÍ2-ÑÍ3 |
t |
0.69-0.76 |
* The multiplicity of the signal: s - single; d - doublet; t - triplet;
m - multiplet.
Multiplets in the signals 2-4 (3.92-5.05 ppm) indicate protons of CH-
and CH2 groups of the glycerol moiety. Thus, the signal 2 shows the presence of two protons at
the second carbon atom, signals 3
and 4 (Fig. 2) indicate the protons
of the first and third carbon atoms of glycerin [6, 8].
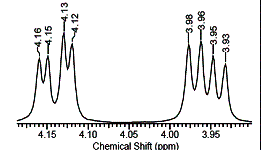
Fig. 2. Signals 3
and 4 in the 1H NMR spectrum of rapeseed oil
In the 1H NMR spectrum in the areas of signal 5-11
(0.65-2.58 ppm) we observe signals for the CH3-, CH2-
allyl protons and fragments of fatty acids.
A signal 5 as a multiplet indicates
the presence of methylene (bis-allylic CH-CH=CH-CH=CH-) protons of lenolic and lenolenic
acids. Signal 10 also points the
presence of lenolenic acid in rapeseed oil [3, 7, 8].
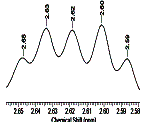
Fig. 3. Signal
area 5 1H -NMR spectrum of rapeseed oil.
The signals 6 and 8 in the 1H-NMR spectra of
oil indicate the protons of acyl-groups, and multiplet of signal 7 indicates the allyl protons of all
unsaturated fatty acids. The signal 9 peaks
show the methylene protons of saturated acyl groups in oleic acid, lenolic and,
lenolenic acids [9]. Doublets of this signal are located in the area 1.09-1.20
(Fig. 1).
Conclusions
Thus, by using the 1H NMR spectroscopy the high content of
polyunsaturated acids (lenolic and lenolenic) in of rapeseed oil was determined.
The high accuracy levels together with the minimal requirements of
sample preparation and short analyses time determine the NMR spectroscopy
method as an ideal technique for rapid and reliable determination of the
authenticity of product.
References
1. Sacchi R., Addeo F., Paolillo L. 1H and 13C NMR
of virgin olive oil. An overview. Magnetic resonance of Chemistry. 1997. 35 (13). S. 133-145.
2. Aparicio R., Aparicio-Ruiz R. Authentication of vegetable oils by
chromatographic techniques. Journal of Chromatography. 2000. 881 (1-2), P. 93-104.
3. Vigli G., Philihidis A., Spyros A., Dais P. Classification of edible
oils by employing 31P and 1H NMR spectroscopy in
combination with multivariate statistical analysis. A proposal for the
detection of seed oil adulteration in virgin olive oils. Journal of
Agricultural and Food Chemistry. 2003. 51 (19). P. 5715-5722.
4. Gromadzka J., Wardencki W. Trends in edible vegetable oils analysis.
Part B. Application of different analytical techniques. Polish Journal of Food
and Nutrition Sciences. 2011. Vol. 61. ¹ 2. P. 89-99.
5. Vlahov G. Application of NMR to the study of olive oil. Prog. NMR
Spectrosc. 1999. 35. P. 341-357.
6. Knothe G., Kenar J.A. Determination of the fatty acid profile by
1H-NMR spectroscopy. European Journal of
Lipid Sciences. 2004. 106. S. 88-96.
7. Chira N., Todaşcǎ C., Nicolescu A., Pǎunescu G.,
Roşca S. Determination of the technical quality induces of vegetable oils
by modern physical techniques. U.P.B. Sci. Bull. Series B. 2009. Vol. 71. Iss.
4. P. 3-32.
8. Guillen M.D., Ruiz A. Edible oils. Discrimination by 1H
nuclear magnetic resonance. Journal of Science of Food and Agriculture. 2003.
83. P. 338-346.