Хімія та хімічні технології/ 6. Органічна хімія
A. I. Panasenko, V. P. Buryak, T. А. Samura, A. S. Gotsulya, S. N. Kulish, V. O. Salionov
Zaporozhye State Medical University
UV ABSORPTION SPECTRA OF «LARUSANUM»
The spectral characteristic of «Larusanum». «Larusanum» is
used in therapeutic practice for the treatment of tuberculosis. «Larusanum» is isonicotinoyl hydrazone furfurilidenacetone.
Therefore pyridine, 4-pyridinecarboxylic acid, furfurilidenacetone may be examined as the models.
Nonbonding electrons in the molecule of pyridine are on one of sp2 hybrid orbitals of nitrogen atom. 2pz atomic orbital is symmetric to z axis, and 2s-atomic
orbital is spherically symmetric. [1]

Therefore n-π * transition in pyridine is allowed. However the value of one-electron
integrals is not big due to the small overlap between 2s- and 2p atomic
orbitals. This explains comparely low intensity of
the n →π*-transition in pyridine. Complete
absorption of n → π*-transition disappears in acid solutions [5]. It is caused with
the formation of a bond between the proton and
nitrogen atoms due to its lone pair of
electrons:

The distinctive feature
of the band of n → π*-transition of pyridine is vibrational structure , which is well
replaced in the vapor spectrum at ~ 180 nm [4]. Furfurilidenacetone is characterized with two bands with
maxima at 233 and 314 nm as a model compound in ethanol solutions (table. 1). The
first
maximum refers
to the "furan"
band, and the second one we consider as the result of p-π-conjugation in chloroform:
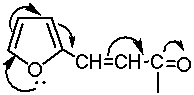
λmaks = 314nm
Besides,
we have established the intense maximum at 263 nm for isoniazid residuum, which
includs in the molecule of «Larusan» (table. 1). According to the
literature [3], the maximum of pyridine in ethanol, is in
the same area (~ 270 nm) but it is low intensity with lg ɛ = 2,65. Thus,
the maximum of model compound (isoniazid)
corresponds to p-π-conjugation
between the pyridine ring and carbonyl group:
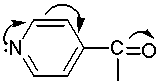
λmaks = 263nm
«Larusanum» is characterized with four absorption
maxima, but for most solvents maxima appear
only in two bands (table. 1). The first band with maxima or bend in the range 221 - 230 nm, is visible for
solutions in concentrated sulphate acid. The second band with a maximum or bend 262 - 275 nm
is observed for solutions in all solvents and corresponds to p-π-conjugation in the 4-piperidilkarbonyl chloroform.
Table 1
Spectral
characteristic of furfurilidenacetone (I), isoniazid (II) and larusanum (III)
|
№ |
Compound |
Solvent |
λ, nm |
ɛ |
lg ɛ |
Electron transfer |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
1. |
I |
95% ethanol |
233 314 |
2190 22910 |
3,34 4,36 |
Furan band p-π- conjugation |
|
2. |
II |
95% ethanol |
263 |
4200 |
3,69 |
p-π- conjugation |
Continuation of Тab. 1
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
3. |
III |
Water |
273 334 |
10230 25120 |
4,01 4,40 |
p-π- conjugation p-π- conjugation |
|
4. |
III |
0,1М NaOH |
275 324 |
Inflection 20890 |
4,32 |
p-π- conjugation p-π- conjugation |
|
5. |
III |
0,1М HCl |
270 321 |
7080 19500 |
3,85 4,29 |
p-π- conjugation p-π- conjugation |
|
6. |
III |
Acetate
buffer solution (pH = 3.85) |
272 325 |
8510 20420 |
3,93 4,31 |
p-π- conjugation furan band |
|
7. |
III |
Conc. H2SO4 |
221 268 384 |
14130 12020 26290 |
4,15 4,08 4,43 |
p-π- conjugation p-π- conjugation p-π- conjugation |
|
8. |
|
95% ethanol |
270 334 |
Inflection 29510 |
4.47 |
p-π- conjugation p-π- conjugation |
|
9. |
|
Cyclohexane |
230 275 331 |
Inflection Inflection 28840 |
4,46 |
p-π- conjugation p-π- conjugation p-π- conjugation |
|
10. |
|
6М NaOH |
275 331 |
Inflection 19050 |
4,28 |
p-π- conjugation p-π- conjugation p-π- conjugation |
|
11. |
|
Dioxane |
270 319 420 |
Inflection 24550 8510 |
4.39 3.93 |
p-π- conjugation p-π- conjugation p-π- conjugation |
|
12. |
|
Chloroform |
262 320 420 |
8510 20420 8510 |
3.93 4.31 3.93 |
p-π- conjugation p-π- conjugation p-π- conjugation |
The
third absorption bands with maxima at 329 - 334 nm is absent only for solutions
in concentrated sulfate acid. The emergence of
this band undoubtedly
is
connected with the p-π-conjugation, in
the result of introduction in the molecule of furfurilidenacetone residuum.
For the third absorption band of «Larusanum», which should be considered the main, bathochromic
shift of the maximum can be observed in the transition from low-polar to a
polar solvent. Thus, maximum is 319 nm in 1,4-dioxane, in chloroform-320 nm in
ethanol and water - at 334 nm (table. 1).
The
fourth band with maximum in the region of 384 - 420 nm is typical only for solutions of
«Larusanum» in concentrated sulfate acid, 1,4-dioxane and chloroform, the
occurrence of which is probably due to the formation of associates of the
"Larusanum -
solvent".
Conclusions
1. UV
absorption spectra of «Larusanum» have been measured in
10 solvents of different polarity (purified water, 0.1 M HCl, 0.1 M NaOH), acetate
buffer solution (pH = 3.85); 95% ethanol, cyclohexane, 6M NaOH, 1,4-dioxane, chloroform.
2. Learning of UV spectra of model compounds (furfurylidenacetone and
isoniazid) makes in ethanol solutions.
3. Four bands of maximum in the region 221 - 230 262 - 275 319 - 334 384
- 420 nm on the spectral curve of «Larusanum»
are observed; the second band of them is the most
typical corresponding to р-π-conjugation in the residue of furfurilidenacetone.
Literature
1. Большаков Г. Ф., Ватаго В. С., Агрест
Ф. Б. Ультрафиолетовые спектры гетероорганических соединений. Л.: Химия, 1969.
– 504 с.
2. Машковский М. Д. Лекарственные средтва.
– Харьков: Торсинг, 1988. – Т. 1 – 560 с., т. 2. – 592 с.
3. Свердлова О. В. Электронные спектры в
органической химии - .:
Химия, 1985. – 248 с.
4. Сильверстейн Р., Басслер Т., Морцил Т.
Спектрометрическая идентификация органических соединений.
М.: « Мир», 1977. – 590 с.
5. Mason
S. F. The electronic spectra of N-heteroaromatic system. Pt II: Substituted
monocyclic azines // J. Chem. Sоc. – 1999. – №5. – Р. 1247 – 1253.