Õèìèÿ è õèìè÷åñêèå
òåõíîëîãèè/5. Ôóíäàìåíòàëüíûå ïðîáëåìû ñîçäàíèÿ íîâûõ ìàòåðèàëîâ è òåõíîëîãèé
Gnatko E.N., Omelchenko A.M., Kravets V.I.
Ukrainian State
Chemico-Technological University, Dnieprpotrovsk, Ukraine
HYDRODYNAMIC REGIME AND MASS TRANSFER COEFFICIENT OF
THE CONCENTRIC CYLINDRICAL FLOWING ELECTROLYZER WITH STATIONARY ELECTRODES
In the diffusion-controlled electrochemical
reactions, the maximal electric current is determined by the mass transfer
coefficient. A larger mass transfer coefficient leads to the higher current
efficiency, due to alleviation of the side reactions, and lower thermal loses. The
main objectives of this publication are to theoretically evaluate the mass
transfer coefficient and, sequentially, the maximal diffusion-limited current
in the concentric cylindrical electrolyzer flowing with stationary electrodes.
The maximal diffusion-limited
current is given by the equation
(1),
![]()
where IL
is the current limited by convective diffusion, z is the valency, F is
the Faraday constant, km
is the mass transport coefficient, which is the complex function of the
reactant’s diffusion coefficient, thickness of unstirred layer of electrolyte
and physical characteristics of electrolyte, A is the effective electrode area, and co is the bulk concentration of the reactant.
From Eq. (1), the maximal value
of diffusion-limited current does not depend on voltage meaning the reaction
rate will attain the maximum at certain voltage and further increase of voltage
will just cause non-productive loses of energy.
Due to
the uncertainty in the effective electrode area, the product of km and A, so-called kmA factor, is usually calculated
by Eq. (1) using experimentally measured value of limited current. The disadvantage
of this method consists in that the determined value is limited to the given
electrochemical reactor.
In more general manner, the
mass transfer coefficient could be theoretically
evaluated of on the basis of analysis of hydrodynamic regime (laminar or
turbulent) of the flow inside the electrolyzer and its geometry. That value of
mass transfer coefficient could be assumed for other similar electrolyzers.
The mass transfer coefficient
is best expressed in terms of dimensionless groups. The Sherwood number (Nusselt
number), characterizing mass transfer, can be used for calculation of
the average mass transfer coefficient kAV
by formula:
![]() (2),
(2),
where ShAV is the average
Sherwood number for entire anode or cathode chambers, D is the diffusion
coefficient of the reactant, and dh is the hydraulic diameter
of the electrolyzer. For the concentric cylindrical reactor de
is calculated by formula [1]:
![]() (3),
(3),
where di and do
are the inner diameter of the outside cylinder and outside diameter of the
inner cylinders, respectively. In our case, dh are 0.24 cm
and 0.21 cm for the anode and cathode chambers, respectively.
The
Sherwood number depends on the hydrodynamic regime inside the reactor and its
geometry, e.g. on the length of the electrodes. The length of the electrodes L in our study is 21 cm. Thus, they can
be considered as long ones, i.e., having a length more than 20dh
(in our case, 4.0 – 4.6 cm). For the long electrodes, the condition of constant
mass flux at the electrode surface is applicable [1]. For the concentric
cylindrical electrolyzer with long stationary electrodes and fully developed laminar flow, the Sherwood number depends
on the aspect ratio r = do/di [1]. Data for ShAV
as a function of r are given in Table 1.
Table 1. Variation of ShAV with aspect
ratio in a long annulus [1]
|
r |
0.8 |
0.6 |
0.4 |
0.2 |
0.1 |
|
ShAV |
5.580 |
5.912 |
6.583 |
8.499 |
11.910 |
The
Reynolds number characterizes
hydrodynamic regime, i.e., laminar or turbulent, of the flow inside the
electrolyzer. It depends on the flow rate, geometry of the reactor and physical
properties of fluid. Experimentally shown that for the Reynolds number about 2400
and below the flow of fluid through a tube is laminar, and turbulence occurs if
the Reynolds number is about 3000 and above. The average Reynolds number ReAV for entire
electrochemical reactor can be calculated using equation [1]:
![]() (4),
(4),
where UAV is the average
linear flow rate in the reactor, cm s-1, and n is the kinematic viscosity of fluid in the
reactor, cm2 s-1. For the calculations, we used n = 10-2 cm2/s, which
is characteristic for the most aqueous solutions.
In
general, the average linear flow rate could vary for anode and cathode chambers
due to their different volumes. The average linear flow rate and the retention
time tR inside the reactor
are given by formulas:
![]() and
and ![]() (5),
(5),
where V is the volume of the anode
or cathode chamber, cm3, and f is the volumetric flow rate,
cm3 s-1.
The
calculated values of the average linear flow rates through the cathode and
anode chambers and the Reynolds numbers are presented in Table 2. The
calculations were based on the following geometric parameters: anode: length – 21 cm, surface area –
52.8 cm2; cathode: length – 21 cm, inner, i.e., electrochemically active, surface area –
88.8 cm2; anode chamber: volume – 7.3 cm3; cathode chamber: volume – 8.4 cm3.
It can be seen that flow inside the electrolyzer
is laminar up to the practically used volumetric flow of 2000 cm3/min,
and values of ShAV presented in Table 1 are applicable for
evaluation of ILAV. Since the aspect ratio r in our
case is ~0.8, the value of ShAV = 5.580 was used for
calculations.
The
value of the maximal diffusion-limited current, averaged over entire chamber ILAV, was calculated by
formula:
Table 2. The Reynolds numbers for the FEM3
reactor for the various flow rates.
|
Volumetric flow rate, cm3/min |
Average linear flow rate, cm/s |
Reynolds number |
||
|
Cathode chamber |
Anode chamber |
Cathode chamber |
Anode chamber |
|
|
100 |
4.15 |
5.04 |
85.40 |
116.02 |
|
200 |
8.29 |
10.08 |
170.81 |
232.04 |
|
300 |
12.43 |
15.13 |
256.21 |
348.06 |
|
400 |
16.58 |
20.18 |
341.62 |
464.07 |
|
500 |
20.72 |
25.22 |
427.02 |
580.09 |
|
600 |
24.87 |
30.27 |
512.42 |
696.11 |
|
700 |
29.02 |
35.31 |
597.83 |
812.13 |
|
800 |
33.17 |
40.35 |
683.23 |
928.15 |
|
900 |
37.31 |
45.40 |
768.64 |
1044.17 |
|
1000 |
41.46 |
50.44 |
854.04 |
1160.19 |
|
1100 |
45.60 |
55.49 |
939.44 |
1276.21 |
|
1200 |
49.75 |
60.53 |
1024.85 |
1392.22 |
|
1300 |
53.90 |
65.57 |
1110.25 |
1508.24 |
|
1400 |
58.04 |
70.62 |
1195.66 |
1624.26 |
|
1500 |
62.19 |
75.66 |
1281.06 |
1740.28 |
|
1600 |
66.33 |
80.71 |
1366.46 |
1856.30 |
|
1700 |
70.48 |
85.75 |
1451.87 |
1972.32 |
|
1800 |
74.62 |
90.80 |
1537.27 |
2088.34 |
|
1900 |
78.77 |
95.84 |
1622.68 |
2204.36 |
|
2000 |
82.92 |
100.89 |
1708.08 |
2320.37 |
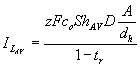 (6)
(6)
where tr is the transport number of the reacting
species. In general, tr is about 0.9 for the reacting species
in both the anode and cathode chambers. For the typical bulk concentration 0.118 M, maximal
diffusion-limited current for the anode is calculated to be 2.8 A. Since water
molecules, which are abundant in the solution, are
discharged on the cathode, the kinetics of
the cathode reaction is not diffusion-limited. Therefore, an overall maximal
current could be evaluated as ~ 3A, since both chambers are connected in
series. This value is at the same order of magnitude with the experimentally
observed currents in our electrolyzer.
Conclusions
1. The flow inside the electrolyzer is
probably laminar in the range of the usually employed volumetric flows of 300 –
600 ml/min.
2. Due to the laminar character of the flow
the mass transfer coefficient does not depend on the volumetric flow rate. In
this case, increasing of the flow rate will lead to decrease of the retention
time and reduce sodium chloride transformation.
Theoretical lowest level of the volumetric
flow rate should be estimated on the basis of actual saline concentration
inside the electrolyzer and the Faraday’s law.
3. To increase the effectiveness of the
mass transfer, the usage of some turbulence-creating devices should be
considered during the design of a new electrochemical reactor.
1. Pickett, D. J. Electrochemical reactor design.
Second edition, 1-529. 1979. Amsterdam, Elsevier. Chemical engineering
monographs. V. 9 (Editor Churchill, S.W.)