UDC 631.436
Thermal
analysis of humic acids of sapropels of the Kondinsky lakes of Khanty-Mansi
Autonomous Area
Shpynova
N. V., Chumak V.A. Sartakov M.P.
Ugra state university, Khanty-Mansiysk,
Russia
Results of the thermal analysis of the humic acids emitted from
sapropels of various lakes, executed with use of modern devices of the
synchronous thermal analysis are given.
Keywords: sapropel, humic acid, thermal analysis.
Use of the modern synchronous
thermal analysis allows with high precision and at a small expense of a sample
to obtain important data on process of thermal decomposition of humic acids of
various genesis [2].
Samples of sapropels for receiving
humic preparations were selected from the top layers of ground deposits of five
various lakes of Khanty-Mansi Autonomous Area located in the Kondinsky area.
Extraction of humic acids was carried out by earlier described technique [1].
The ash-content of preparations doesn't exceed 2,28%.
The thermal analysis was carried out
on the thermogravimetric TGA/SDTA 851e analyzer (firm METTLER TOLEDO STAR,
Germany) at a free access of air to oven space.
The Differential Scanning Curves
(DSC) of the chart of humic acids of sapropels, apparently from figure 1, give
an idea of the thermal effects which are taking place at high-temperature
oxidizing decomposition of these samples.
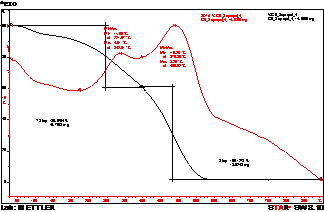
Ðèñóíîê 1 – the DSK-chart of humic acid of sapropel
It is easy to notice that
thermal destruction causes a row ekzo-and the endothermic effects testifying to
gradual destruction of a molecule. In its structure two sharply various parts
on thermal stability are distinctly allocated: the nuclear aromatic (is
steadier) also lateral aliphatic chains for which smaller heat stability is
characteristic considerably. High intensity of exothermic reactions between 500
and 600 0C is caused by destruction of nuclear part, endothermic and exothermic
effects in the field of low temperatures (60 – 400 0C) are connected with
changes and gradual destruction of peripheral part.
Sizes of warmth of combustion
depend on degree of a benzoidnost of humic acids of sapropels. The division of
thermal reactions of destruction of humic acids on the low-temperature and
high-temperature areas, Z which were characterized by an indicator, allows to
reveal structural features of humic acids of a various origin (tab. 1).
Table 1 - Data of TGA, set of
humic acids of sapropels of Srednego Priobya
|
sample No. |
Weight loss in low-temperature
area (0 - 4000Ñ) |
Weight loss in
high-temperature area (400-7000Ñ) |
Z |
Ash-content, % |
|
1 |
35,14 |
62,83 |
0,56 |
2,20 |
|
2 |
39,53 |
59,13 |
0,67 |
1,50 |
|
3 |
38,81 |
60,03 |
0,65 |
1,21 |
|
4 |
35,28 |
62,74 |
0,56 |
2,28 |
|
5 |
36,10 |
62,81 |
0,57 |
1,12 |
Note: Z – the relation of loss
of weight in low-temperature area to weight loss in high-temperature area.
Apparently from given tables,
this indicator for group of companies of sapropels changes from 0,56 to 0,67
with increase in extent of decomposition of an initial organic substratum that
is more expressed at sample No. 3 group of companies.
Reduction of coefficient of Z
happens at humic acids to smaller molecular weight at the expense of intensive
destruction of mostikovy groups at relative accumulation of stable groups. Thus
stable groups of low-molecular fractions become much less steady.
As on òåðìîãðàììàõ by us the
average maximum temperature of thermoeffect in high-temperature area which made
535,4 0C, and average maximum temperature in the low-temperature
area, equal 346,4 0C is noted.
It should be noted that with a
various molecular weight the general principle of a structure of humic acids
remains. from the point of view of heat stability separate fractions of. To.
are presented by identical fragments for various soils and substrata of the
Ob-Irtysh flood plain.
Using data thermoweight humic
acids, probably, would be to present in details process of thermal destruction
and experimentally to prove each of its stages if idea of a structural
structure of humic acids were fuller.
According to charts
ascertaining of changes in chemical structure is possible only, and about the
direction of process it is possible to assume, considering data of structural
chemistry in a complex with other spectral and chemical methods.
Conclusions
1. For the first time data on
thermal stability of humic acids of sapropels of the Kondinsky Area of
Khanty-Mansi Autonomous Area created on lakes of various type are obtained.
2. Thermal stability of humic
acids of the studied objects differs, but, generally, this is characterized by
existence typical for all preparations and ýêçî thermoeffects that confirms
presence of identical structural fragments.
Literature
1. Commissioners I.D.Vliyaniye
of a way of extraction of humic acids from raw materials on a chemical
composition of received preparations / I.D. Commissioners,
I.N.Streltsova//Nauchn. Òð. Tyumen SHI. – Tyumen, 1971. - T.14. – Page 34-48.
2 . Tikhova V.D. Use of the
modern thermal analysis for research of humic acids of peat / EL of Tikhov, L.
S. Sartakov, I.D.Komissarov//Humic substances in the biosphere: ñá. íàó÷. works
IV of the All-Russian conference. – M: Moscow State University publishing
house, 2007. – Page 203-207.