Ogurtsova1
V.V., Zhytniakivska1 O.A., Trusova1 V.M., Gorbenko1
G.P,
Kirilova2 E.M., Kirilov2 G.K., Kalnina2 I.
1V.N. Karazin
Kharkiv National University, Ukraine
2Daugavpils
University, Latvia
Interactions between a new fluorescent benzanthrone dye and model membranes
Due to their versatility luminescent
techniques are widely used in biophysics studies, particularly for the covalent
and noncovalent labeling of biological objects, including natural and model
membranes. High sensitivity
of fluorescent probes to the environment provokes strong prerequisites for their use as markers in probing the
membrane structure and
protein-lipid interactions.
Among the organic luminophores, which localize in the hydrophobic region of liposomes are benzanthrone
dyes composed of 3-methoxybenzanthrone. Because of their bright fluorescence, and color characteristics these probes are widely used as luminescent pigments and daylight components in lasers [1, 2].
|
|
|
|
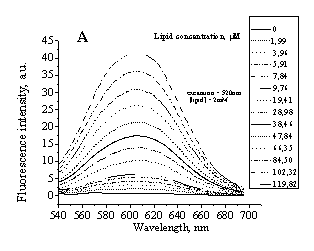
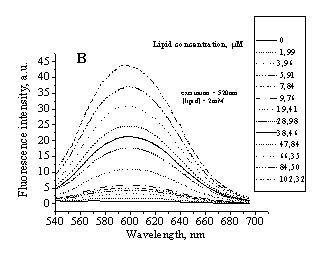
Fig 1. Typical emission spectra of IAH in PC (A)
liposomes and PC/Chol (30%) liposomes (B). Excitation wavelength was 520 nm. |
|
The
purpose of this work was to investigate the
sensitivity
of a new benzanthrone
dye, referred here as
IAH, to the changes in
membrane environment. For this purpose the method of fluorescence spectroscopy was
used. Firstly the partition coefficients of the dye in the lipid phase were
measured by titration of the probe IAH with liposomes, which composed of
phosphatidylcholine (PC) and its mixtures with cholesterol (PC/Chol) and
cardiolipin (PC/CL). Liposomes were prepared by extrusion technique [3]. The typical
fluorescence spectra of this dye are represented in Fig.1.
To
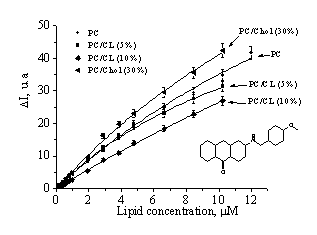
characterize IAH-lipid interaction more detail, we determined the dye partition
coefficients (Kp) for different lipid systems by analyzing the binding
isotherms, presented in Fig.2.
|
|
Table 1.
Quantitative parameters of the dye-lipid binding |
||
|
System |
Partition coefficient |
Quantum yield |
|
|
PC |
3474±578 |
0.06 |
|
|
PC / CL 5% |
6556±380 |
0.05 |
|
|
PC / CL 10% |
1464±236 |
0.04 |
|
|
Fig.2. Fluorescence intensity increase as a function
of lipid concentration |
PC / Chol 30% |
5584±868 |
0.07 |
As
seen in Table 1, inclusion of sterol Chol into PC bilayer give rise to
increase Kp and fluorescence quantum yield relative to the neat PC
membrane. Such
effects can be interpreted in terms of the appearance
of additional packing defects in the interfacial bilayer region on Chol
addition. It is assumed that the changes in lipid packing
density on Chol inclusion allow a greater number of water molecules to
penetrate in the headgroup bilayer region, which,
in turn, brings about the increase
of partition coefficient compared to the neat PC membrane.
In CL-containing
systems partitioning coefficient was found to exhibit unambiguous behavior (it
has a tendency to increase in PC/CL (5%) and decrease in PC/CL (10%)), when
the fluorescence quantum yield of dye IAH decrease relatively to the neat PC
membrane. Such quantum yield decrease can be explained
by the higher level of CL oxidation (oxidative index~1), which favors
enchanced water penetration into the membrane interior. Unambiguouse behavior
of Kp in CL-containing systems can be interpreted
in terms of specific conical structure of CL molecule.
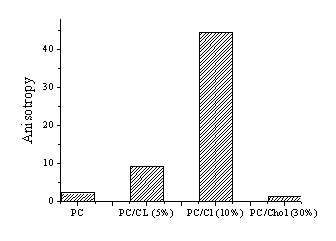
At the next step
of the study the fluorescence anisotropy of IAH in different lipid systems
were measured by adding to the liposome-containing systems a native protein
lysozyme. Present study shows that inclusion of lisozyme to PC/CL (10%)
membrane give rise to increase of the fluorescence anisotropy of the dye IAH.
It can be explained in terms of electrostatic interactions between the anionic
lipid cardiolipin and opposite charged lisozyme.
|
|
Fig.3 Fluorescent anisotropy after addition of native protein lisozyme. |
In conclusion, the present study
demonstrated that the examined dye IAH displays high lipid-associating
ability. It was found that partition coefficient of IAH increases upon
inclusion of cholesterol into phosphatidylcholine bilayer. The obtained
results suggest that benzanthrone dyes can be effectively used as markers of
physicochemical properties of the biological objects.
References
1.
Dobretsov G.E. Fluorescent probes in studying cell membranes and lipoproteins//M.:Nauka.1989.
2.
Vladimirov Y.A., Potapenko A.Y. Physico-chemical bases of photobiological
processes //M.: Drofa, 2006.
3.
Mui B., L. Chow L., Hope M.J. Extrusion technique to generate liposomes of
defined size //Meth. Enzymol. 2003. V. 37, P. 3-14.