E. Kukhar, V. Kiyan,
A. Khalikova
S.
Seifullin Kazakh Agro Technical University,
Astana, Kazakhstan
IMMUNOCHEMICAL CHARACTERISTICS ANTIGENS
DERMATOMYCETES MICROSPORUM CANIS
Microsporia - (from
the Greek. mikrós – small and sporo – seed
sowing), microsporoz, ringworm, a contagious disease (mycosis) of animals, caused by fungi
Microsporum, characterized by lesions
of the skin and its derivatives. Microsporia is one of the most common
dermatological pathologies in domestic animals. Microsporia get sick more often
cats, dogs, fur-bearing animals, rabbits, at least - horses, sheep, goats,
pigs, deer, monkeys, tigers. The problem of Microsporia, caused
by zooantropogenic mushrooms, is still one of the urgent problems of modern
dermatomycology in connection with the observed epidemic outbreaks of epizootic
in humans and domestic animals in several countries [1].
In Kazakhstan, the
incidence of zooantroponosis microsporia of the Republic of the public was in
the 90s. up to 42,6-44,3%. According to the Ministry of Health the incidence of
dermatomycoses in Kazakhstan is a stable level, while microsporia registers
everywhere. Intensive microsporia incidence in 2007 was 79.8 per 100 thousand
population (Figure 1).
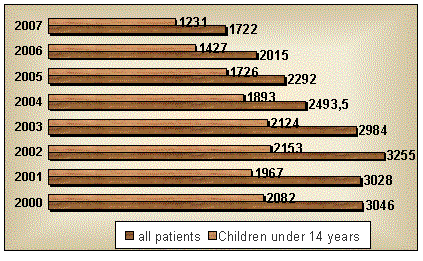

Figure 1 - Incidence microsporia population of Kazakhstan for 2000-2007
(the
figures indicate the number of patients)
At present,
diagnosis of dermatomycoses, includes methods such as microscopy of
pathological material, cultural and fluorescent diagnostics. These methods do
not allow a fairly high degree of accuracy the correct diagnosis, not to
mention the specific identification of the pathogen. Traditional diagnostic
methods do not meet modern requirements, and highly effective methods of
diagnosis of pathogenic dermatomycoses in Kazakhstan has not yet been
developed. Therefore, before the medical and veterinary mycology is an issue on
the development of more sophisticated methods that allow to quickly diagnose
fungal infections.
One of the important
areas of modern biotechnology, both in this country and abroad, is the
development and improvement of technological processes of production of
diagnostic biologics against zooantroponosis disease [2].
These requirements
are fully in line immunoassay diagnostic test kits. In order to develop
diagnostic test systems are required antigens that differ high specificity and
activity. Thus, the aim of our study was to obtain antigenically-active
components of the cell wall dermatomycetes Microsporum
canis and the study of their
immunological properties.
Methods and
materials
In the experimental
work was used dermatomycetes strain of M.
canis, isolated from pathological material. In order to obtain
antigenically-active components of the cell walls of submerged cultivation was
carried out dermatomycetes M. canis
in 500 ml flasks on Sabouraud nutrient mediums not
within 15 days.
As the antigens were
used: protein components of the cell wall dermatomycetes M. canis, isolated in L.
Tabatabai (1979) (antigen ¹1); protein components of the cell wall dermatomycetes M. canis, isolated by the method of
freezing and thawing (antigen ¹2); culture medium antigen (CM). The antigens were purified by low-speed
centrifugation and gel-filtration chromatography [3].
Gel-filtration
chromatography columnar separation of protein antigens was performed on the
equipment of the company "Rharmacia" in the sorbent in the quality
used vehicle brand Sephadex and Sephacryl (“Sigma”) [4].
The degree of
purification of antigens was monitored by polyacrylamide gel electrophoresis by
the method of V.K. Laemmli (1970) on
the unit to a vertical electrophoresis («Compact dual mini code», England)
using a Tris-glycine buffer, and 12.5% polyacrylamide gel in the
presence of sodium dodecyl sulfate.
To evaluate the
antigenic activity of protein antigens using immunoblotting method, the
reaction RMA, IDR and indirect ELISA.
Results of the study
Biomass dermatomycetes
got deep cultivation within two weeks. At the end of the growth of mycelial
mass was separated from the liquid medium by filtration through a paper filter
and used to isolate antigenically-active components.
The average yield of
biomass dermatomycetes M. canis was
5.2 g per 100 ml of liquid medium (Figure 2).
![]()
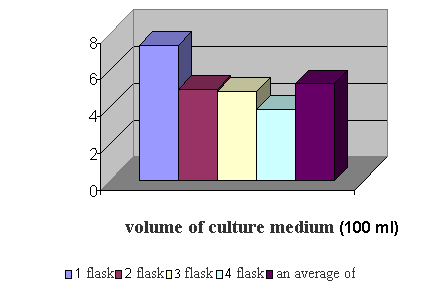

Figure 2 - The yield of biomass
dermatomycetes M. canis
Output antigen ¹1 was 0.375 mg, and antigen ¹2 – 0.325 mg with 1 g of fungal biomass. In the obtained preparations was
determined the concentration of proteins and carbohydrates.
The result is a
visually into account (Table 1).
Table 1 -
Characteristics of antigens dermatomycetes M.
canis
|
The antigen |
Concentration (in mg/ml) |
Ratio carbohydrates |
|
|
Carbohydrates |
Proteins |
||
|
Antigen ¹1 |
0,005 |
0,250 |
1:50 |
|
Antigen ¹2 |
0,025 |
0,250 |
1:10 |
|
Culture medium antigen (ÑM) |
1,0 |
0,030 |
1:0,03 |
Further analysis of
the antigens was performed by column chromatography, gel-equipment of «Pharmacia». As the sorbent used Sephadex
G-25 and Sephacryl S-200, as eluate – 0.9% NaCl solution and 0,02 M Tris-HCl.
The results of the
separation of antigens in samples of gel-filtration column chromatography, the
separation into fractions were recorded on an automatic recorder (Figure 3).
![]()
![]()
![]()
![]()
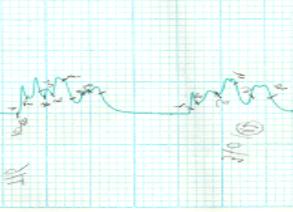
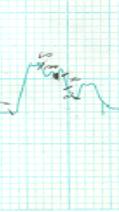
1 2 3
Note:
1 – antigen ¹1, 2 – antigen ¹2, 3 – antigen-CM
Figure 3 – Chromatogram of purification of antigens dermatomycetes
M. canis
As seen in Figure 3,
the cell wall protein antigens of M. canis
contain six, The culture of the antigen contains four components are well separated
into fractions.
Analysis of protein antigens was performed using polyacrylamide gel
electrophoresis by the method of V.K.
Laemmli (1970) on the vertical electrophoresis device
(Hoefer Scientific, USA) using a Tris-glycine buffer, 12% polyacrylamide gel
in the presence of sodium dodecyl sulfate at capacity – 120 V, a current - 30
mA, voltage – 50W.
The resulting
protein fraction had the following molecular weights: a protein antigen is ¹1 – 116 kDa, 110 kDa, 98 kDa and 36 kDa; protein antigen ¹2 – 116 kDa, 110 kDa, 98 kDa and 66 kDa, 36 kDa; the
antigen-CM – 110 kDa (Figure 3).
Electrophoregram dermatomycetes
protein antigens were transferred to nitrocellulose membrane (Schleicher & Schuller, Germany) for
3 h at a power of - 120 V, a current – 45 mA, voltage – 50W by means of the
device company Scientific (USA) by
the method of H. Towbin et al.
Free media sites
blocked with 1% solution of bovine serum albumin. Nitrocellulose membrane was washed
and then incubated for 1 hour with the patient's blood serum microsporia man,
and then kept in a working dilution of antibodies against human immunoglobulins
labeled with horseradish peroxidase. Then performed immunochemical
manifestation of a nitrocellulose membrane, which plunged it into the substrate
solution, prepared immediately before use.
A positive reaction was characterized by the appearance of lines, painted in
gray-purple color (Figure 4).
30 êÄà 14 êÄà 20 êÄà 24 êÄà 32 êÄà 45 êÄà 66 êÄà


1
2
3 4
Note: 1 – markers, 2 – antigen ¹1; 3 –
antigen ¹2, 4 – antigen
Figure 4 – Results of
immunoblotting of protein antigens
dermatomycetes M. canis
As seen in Figure 4,
the molecular weight antigenic protein antigen fraction number 1 that interacts
with the antibody is 30 kDa.
To study the
specific activity of antigen immunized albino mice. In further studies, we
tested antigens derived on the activity and specificity with the sera of mice
in indirect ELISA.
We found that both
the antigen and immunogenes quite active. They identify specific antibodies by
indirect ELISA in sera diluted 1:800
(antigen ¹1) and 1:1600 (antigen ¹2).
The presence of
antigen precipitating properties dermatomycetes M. canis was studied in the reaction
of immunodiffusion (IDR). As a result, we found that the precipitating antigen ¹1 has properties of that was
characterized by the appearance of a clear precipitin line with the
corresponding immune serum (Figure 5).
![]()
![]()
![]()
![]()
![]()
![]()
![]()
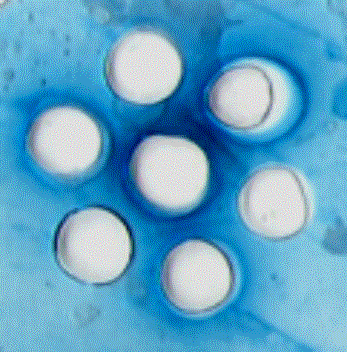
AG – the antigen ¹1 M. canis; 1 – mouse serum
immunized with the antigen ¹1; 2 – mouse serum immunized with the antigen ¹2, 3 – mouse serum immunized with the antigen-CM; 4 - mouse serum immunized with the protein antigen T. verrucosum; 5 –
serum mice, immunized with LTP-130; 6 – negative control.
Figure 5 – The reaction of
immunodiffusion in agarose gel protein
antigen ¹1 dermatomycetes
M. canis
To identify the
properties of the obtained agglutinated antigen reacted droplet agglutination
and micro agglutination reaction.
In the reaction of
agglutination (RA) on glass droplet formation characteristic of flakes was
observed after 3-5 seconds after the introduction of serum. This indicates the
presence of marked agglutinating properties of the derived antigens.
In setting up the
reaction with micro agglutination (RMA) derived antigens, the titer of specific
antibodies detected in relatively high dilutions of sera (Table 2).
Table 2 - Results of testing sera of people in the micro agglutination reaction
with protein antigens of M. canis
|
Antigens |
Antibody titers of sera
of blood |
||
|
Ê + |
Ê - |
Ê+ to T. verrucosum |
|
|
Protein antigen ¹1 |
1:1024 |
ÐÎ |
1:2 |
|
Protein antigen ¹2 |
1:512 |
ÐÎ |
1:16 |
|
Notes: K+ – human serum,
with a clinically confirmed diagnosis of Microsporia,
K- – a negative control |
|||
As can be seen from
Table 2, agglutinating antibodies were found in the RMA at a dilution of 1:1024
sera before – with the protein antigen ¹1, 1:512 – to a protein antigen ¹2.
Antigenic activity
of antigenic preparations obtained by us in a indirect ELISA, IDR, RMA and RA
is presented in Table 3.
Table 3 – Results of the study of antigenic properties of antigens dermatomycetes
M.canis in various serological tests n = 3, P<0.05
|
Serological |
Of antibody titers
in sera, identified antigens of the fungus M. canis |
||
|
Protein
antigen
¹1 |
Protein
antigen
¹ 2 |
Antigen-ÑM |
|
|
IDR |
+ |
- |
- |
|
ÐÀ |
+ |
+ |
- |
|
RMA |
1:1024 |
1:512 |
1:2 |
|
Indirect
ELISA |
1:1200
± 0,25 |
1:1200
± 0,25 |
1:100
± 0,2 |
From Table 3 it can
be concluded that the isolated protein antigeny fungus M.canis, have distinct antigenic properties. The maximum titer of
antibodies detectable protein antigens in the ELISA is 1:1600, 1:1024 and PMA.
Conclusions
Thus, the
investigation resulted in two protein antigen of M. canis. A study of immunochemical characteristics of antigens
dermatomycetes revealed the presence of sufficient activity in the ELISA and
PMA, as well as the presence of precipitating properties.
References:
1.
Pozdnyakov, O.N., Makhnovets, E.N., Reshetnikov T.B.,
Nemchaninova O. Epidemiology of zooantropophyles dermatomycoses in Novosibirsk
// Problems of medical Mycology. – St-P., 2003. – V.5,
¹2. – S. 64.
2.
Yelinov, N.P., Vasilyeva N.V., Raznatovsky K.I. Ringworm,
or tinea surface of the skin and its appendages - the hair and nails.
Laboratory diagnosis of // Problems of medical Mycology.
– St-P., 2008. – T.10, ¹1. – S. 27-34.
3.
Toleutaeva. S.T. Manufacturing technology of antigens
from cultures of dermatophytes for the production of serological reactions //
Actual problems in the diagnosis of animal diseases: Sat. Mater. II Int.
Scientific-Practical. Conference. – Almaty, 2005. – S. 325-326.
4.
Osterman, L.A., Chromatography of proteins and nucleic
acids. – Moscow: Nauka, 1985. – S. 65-91, 145-166.