Egorov N.B.
Department of Applied Chemistry of Rare, Scattered and Radioactive Elements Institute of Physics and Technology Tomsk Polytechnic University, Russian Federation
THE SYNTHESIS OF SODIUM TETRATHIOSULFATOPLUMBATE HEXAHYDRATE
The interaction between equivalent amounts of Pb(NO3)2
or Pb(CH3COO)2 with Na2S2O3 in
aqueous solutions yields poorly soluble compounds of PbS2O3
[1] and Pb3(S2O3)2(CH3COO)2
[2]. These compounds are dissolved in an excess of Na2S2O3
to form lead thiosulfate complex ions [Pb(S2O3)2]2-,
[Pb(S2O3)3]4-, and [Pb(S2O3)4]6-.
The purpose of this work
was to recover water-soluble lead thiosulfate complexes in the solid phase and
to characterize them.
The following reagents
were used: Na2S2O3×5H2O
(Aldrich), Pb(NO3)2 (Aldrich), and PbS2O3,
prepared by reacting Pb(NO3)2 with Na2S2O3
solutions [1].
Lead thiosulfate
complexes were synthesized using two methods. In method 1, precisely weighed
portions of Na2S2O3×5H2O
and Pb(NO3)2 were dissolved in a minimum volume of water
and mixed by adding Pb(NO3)2 solution to a Na2S2O3
solution; the relevant reaction was:
Pb(NO3)2+xNa2S2O3→Na(2x-2)[Pb(S2O3)x]+2NaNO3, (1)
where
x =
2 - 4.
Reaction 1 proceeded in
two stages: at first, poorly soluble PbS2O3 was formed in
the solution (the solubility of the product was 4×10-7),
and then it was dissolved in an excess of Na2S2O3.
In method 2, a PbS2O3
powder was dissolved in a saturated solution of Na2S2O3;
the corresponding reaction was:
PbS2O3+yNa2S2O3→Na2y[Pb(S2O3)(y + 1)], (2)
where y = 1 - 3.
The PbS2O3
formed in solution and mixed according to method 1 and the PbS2O3
powder used in method 2 could only be completely dissolved when the molar ratio
of Pb2+:S2O32- was equal to 1:4 or
higher. At other ratios, insoluble PbS2O3 remained in
solution.
Thus, the lead
thiosulfate complexes were recovered to the solid phase from solutions with a
molar ratio of Pb2+:S2O32-= 1:4.
On heating and storage
the lead thiosulfate complex solutions decomposed to form PbS and did not allow
their isolation by evaporation of the solvent.
The lead thiosulfate
complexes were isolated from solution using alcohol. When ethanol was used, the
lead thiosulfate complexes were isolated as an oily liquid, which was separated
and treated with absolute ethanol (without additional treatment, the liquid
decomposed over time to form PbS). The oily liquid gradually transformed into a
powder, which was filtered, washed, and dried in air.
A weak point of method 1
was the necessity to reprecipitate the product due to NaNO3
contamination, which promoted decomposition of the lead thiosulfate complexes.
In this case, the product yield decreased.
The product obtained was
a non-hygroscopic white powder. The yield of the lead thiosulfate complexes in
method 2 was 75%.
The complexes were
analyzed for lead [3] and sulfur [4]. The amount of water was determined from
thermal analysis data.
The anal. calc. (%) for Na6[Pb(S2O3)4]×6H2O:
Pb, 22.98; S, 28.45; H2O, 11.98.
Found (%): Pb, 23.1;
23.02; S, 28.40; 28.35; H2O, 11.81.
Thus, according to the
data from the elemental and thermal analyses, the compound synthesized was
formulated as Na6[Pb(S2O3)4]×6H2O
(sodium tetrathiosulfatoplumbate hexahydrate).
X-Ray powder diffraction
patterns were measured on a Shimadzu XRD 6000 diffractometer (CuKa radiation,
l = 0.154056 nm).
The X-ray phase study
showed that the compounds synthesized by methods 1 and 2 were alike, being one
individual substance that possessed a characteristic X-ray pattern that
contained no lines from the original components (Figure 1).
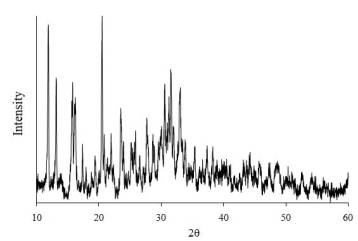
Figure
1. The
XRD pattern of the Na6[Pb(S2O3)4]×6H2O
The compound synthesized
was highly soluble in water. At concentrations of 0,5 M and higher, Na6[Pb(S2O3)4]×6H2O
dissolved with the formation of a minor amount of PbS, and the solution turned
brown. On storage, the highly concentrated solutions decomposed over time to
form PbS.
At a solution
concentration of 0,1 M, pearly disk-shaped crystals precipitated from the
solutions after 8 - 10 h. The IR spectrum of the compound isolated from the solution
was identical to that of PbS2O3. This indicates that the
complex undergoes hydrolysis and decomposes in an aqueous solution.
It should be noted that
the PbS2O3 prepared by a reaction between solutions of
Pb(NO3)2 and Na2S2O3 had
no pronounced crystal structure, unlike the PbS2O3 formed
by the hydrolysis of Na6[Pb(S2O3)4]×6H2O.
References:
1. Freedman
A.N., Straughan B.P. // Spectrochim. Acta. 1971. A 27. P. 1455–1465.
2.
Norlund C. A.; Harell R. G. // Acta Chem. Scand. 1990. 44. P. 1077–1079.
3. Schwarzenbach
G.; Flaschka H. Die komplexometrische Titration. Ferdinand Enke: Stuttgart,
1965.
4. Busev A.I.; Simonova L.N. The Analytical Chemistry of Sulfur. Nauka: Moscow, 1975.