UDK
547.792ʼ532ʼ367ʼ857.4.03/.04.057
Gotsulya A. S., Samko A. V., Panasenko O. I.
Zaporozhye State Medical University
Synthesis,
transformations and physic-chemical properties of
4-(2-methoxyphenyl)-5-R-1,2,4-triazole-3-thiones and 4-(2-methoxyphenyl)-3-R-alkyl-,
(aryl-, heterylthyo-)-4H-1,2,4-triazoles
Keywords: theophylline, 1,2,4-triazole,
physic-chemical properties
Annotation. Researched reactions of obtaining
4-(2-methoxyphenyl)-5-R-1,2,4-triazole-3-thiones and
4-(2-methoxyphenyl)-3-R-alkyl-, (aryl-, heterylthyo-)-4N-1,2,4-triazoles (R =
CH3, C6H5). Studied the physic-chemical
properties of compounds.
Introduction.
Alkylthyoderivatives of heterocyclic compounds possess a wide range of
pharmacological properties. They manifest neuroprotective, restorative,
anti-hypoxic, antioxidant and immunomodulation activity [1]. Therefore,
research this group of compounds causes a great interest.
The purpose of the work. Synthesis and research of physical and chemical properties of
4-(2-methoxyphenyl)-5-R-1,2,4-triazoles-3-thiones and
4-(2-methoxyphenyl)-3-R-alkyl (aryl-, heterylthyo)-4N-1,2,4-triazoles (R = CH3,
C6H5).
Materials and methods. Research of physical and chemical properties of obtained compounds performed,
using methods that are listed in the State Pharmacopoeia of Ukraine. The
melting point determined by open capillary method on the device PTP (M). The
structure of compounds was confirmed by elemental analysis on the Elementar
Vario L cube (CHNS) device, IR-spectra (4000 - 400 cm-1) were
remowed on a module of the ALPHA-T spectrometer Bruker ALPHA FT-IR. 1H
NMR spectra of compounds were recorded, using a spectrometer «Mercury 400»
(solvent - DMSO-d6, internal standard - tetrametylsylan). Chromato-mass spectral researches performed on the instrument Agilent
1100 Series LC/MSD System, ionization method - chemical ionization at
atmospheric pressure (APCI).
As
initial materials for the synthesis of S-alkyl, heteryl-, arylderivatives of
1,2,4-triazole-3-thiones we used 4-(2-methoxyphenyl)-5-methyl-1,2,4-triazole-3-thione
(2.9) and 4-(2-methoxyphenyl)-5-phenyl-1,2,4-triazole-3-thione (2.10). These
thiones we obtained, using already known methods in the literature [1, 5]. Initial compounds for the synthesis of
4-(2-methoxy-phenyl)-5-methyl-1,2,4-triazole-3-thione (2.9) and
4-(2-methoxyphenyl)-5-phenyl-1,2,4-triazole-3-thione (2.10) we used carbon (IV)
sulfide, ammonia and 2-methoxyaniline [2]. Due to the interaction of ethyl
acetate, hydrazine hydrate and butylbenzoate in ethanol medium were synthesized
corresponding hydrazides. Obtained hydrazides react with 2-methoxy-phenylizothyocyonate,
obtained on the first stage, with a formation of
2-acetyl-N-(2-methoxyphenyl)hydrazyncarbothyoamide (2.3) or
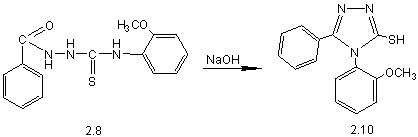
2-benzoyl-N-(2-methoxy-phenyl)-hydrazyncarbothyoamide (2.8) (pic. 1), by means
of which in alkaline medium received thiones 2.9 and 2.10.
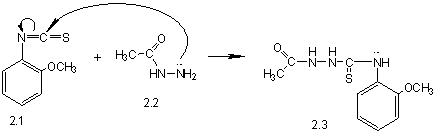
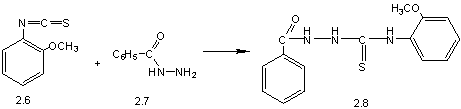
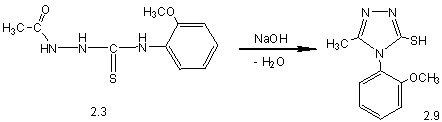
R
= CH3, C6H5
Pic. 1. Synthesis of 4-(2-methoxyphenyl)-5-R-1,2,4-triazole-3-thiones
Table 1
4-(2-methoxyphenyl)-5-R-1,2,4-triazole-3-thione
(2.9, 2.10)
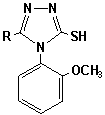
|
Compound |
R |
Ò m., °Ñ |
Gross-formula |
Yield, % |
|
2.9 |
H |
208 - 210 |
Ñ10Í11N3ÎS |
77 |
|
2.10 |
Ñ6Í5 |
218 - 219 |
Ñ15Í13N3ÎS |
46 |
Contin. Table 1
|
Compound |
Found, % |
Calculated, % |
||||||
|
C |
H |
N |
S |
C |
H |
N |
S |
|
|
2.9 |
54,29 |
5,03 |
18,97 |
14,52 |
54,28 |
5,01 |
18,99 |
14,49 |
|
2.10 |
63,59 |
4,65 |
14,82 |
11,33 |
63,58 |
4,62 |
14,83 |
11,32 |
Individuality
of synthesized compounds (Table. 1) confirmed by thin layer chromatography
method in different solvent’s systems (Table. 2).
IR-spectrum
of compounds 2.9 and 2.10 characterized by the presence of distinct bands of
valence vibrations of average intensity bonds of C-H aromatic ring (νcH>
3054 cm-1). Absorption in 1604 - 1469 cm-1 area in the
spectra of these compounds in the form of four bands and intense absorption in
spectra, which situated in the area below 974 cm-1 also, confirms
the presence of an aromatic fragment. Planar
deformation C-H vibrations in areas 1049 - 1012 cm-1 and 775 cm-1
-745 in it’s turn prove the presence of 2-methoxyphenyle fragment.
Additionally, available bands of methoxygroup vibrations within 2847 - 2850 cm-1.
Characteristic absorption band of valence vibrations of compounds 2.9 and 2.10
in area 2757 - 2723 cm-1 caused by the presence of SH-group.
In
order to establish the possible existence of thione-tiole tautomerism were
studied electronic spectra of 4-(2-methoxyphenyl)-5-methyl-1,2,4-triazole-3-thione
(2.9) and 4-(2-methoxyphenyl)-5-phenyl-1,2,4-triazole-3-thione (2.10). [7]
UV-spectra
measured in solvents of different polarity: water, 95% ethanol, 0.1 M HCl, 1 M
H2SO4, 0.1 M NaOH, n-hexane [7]. Measurements performed,
using the device SPECORD 200-222U214 in quartz cuvettes with a thickness of
working layer 1 cm. Observed electronic spectra of researched substances
characterized in all cases by three absorption bands, which have three - five
maximums.
Chemical properties, namely, the interaction of
various derivatives of 1,2,4-triazole-3-thione with halogenalkanes, described
in domestic and foreign works [3, 4].
Considering the limited amount of published
information about the alkylation of 1,2,4-triazole-3-thiones in which the
substituent in the fourth position is a nucleus of 2-methoxyphenole, and in the
fifth - methyl or phenyl radicals, we established the aim to receive a number
of alkyl- , aryl- and heterylderivatives of this heterocyclic system.
Alkylation, arylation, heterylation of
4-(2-methoxyphenyl)-5-methyl-1,2,4-triazole-3-thione (2.9) and
4-(2-methoxyphenyl)-5-phenyl-1,2,4-triazol-3-thione (2.10) haloide alkanes
(propilbromide, alilchloryde, amylbromide, nonyl-bromide, decylchloryde) halogenaryles
(benzylchloryde, phenetylchloryde, 2-nitrochlorbenzene, 4-nitrochlorbenzene)
halogenheterocycles (chlorpyrydine, 2-chlorhinoline) halohentcycloalkanes
(cyklohexylchloryde) performed in ethanol medium with the presence of
ekvimolecular amount of sodium hydroxide. The reaction mixture heated up to a
neutral environment, filtered, the solvent evaporated, obtained compounds
2.11-2.29.
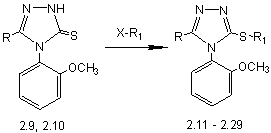
R= ÑÍ3,
Ñ6Í5; R1= Alk, Ar, Het
Pic. 4. Alkylation, arylation, heterylation of
4-(2-methoxyphenyl)-5-R-1,2,4-triazole-3-thione (2.9, 2.10)
Chemical properties, namely, the interaction of
various derivatives of 1,2,4-triazole-3-thione with halogenalkanes described in
domestic and foreign works [2, 3]. Considering the limited amount of literature
information about the alkylation of 1,2,4-triazole-3-thiones in which a
substituent in the fourth position is a nucleous of 2-methoxyphenole, and in
fifth - methyl or phenyl radicals, we established the aim to get a number of
alkyl- , aryl- and heteryderivatives of this heterocyclic system. Alkylation, arylation, heterylation
of 4-(2-methoxyphenyl)-5-methyl-1,2,4-triazole-3-thione (2.9) and
4-(2-methoxyphenyl)-5-phenyl-1,2,4-triazol-3-thione (2.10) by haloide alkanes
(propylbromide, alylchloryde, amilbromide, nonilbromide, decylchloryde) by
halogenaryles (benzylchloryde, phenetylchloryde, 2-nitrochlorbenzene,
4-nitrochlorbenzene) by halogenheterocycles (chlorpyrydyne, 2-chlorchinoline)
by halogencycloalkanes (cyclohexylchloryde ) we performed in ethanol medium in a presence of equimoleqular amount
of sodium hydroxide. The reaction mixture heated to a neutral environment,
filtered, the solvent evaporated, obtained compounds 2.11-2.29.
Table 2
4-(2-methoxyphenyl)-3-alkyl-,
(aryl-, heterylthyo-)-5-R-4H-1,2,4-triazoles
(2.11 - 2.29)
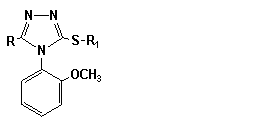
|
Ñompound |
R |
R1 |
Ò m., ºÑ |
Brutto-formula |
Output, % |
|
|
1 |
2 |
3 |
4 |
5 |
6 |
|
|
2.11 |
CH3 |
methyl |
68 - 70 |
Ñ13Í17N3ÎS |
64 |
|
|
2.12 |
CH3 |
amyl |
73 - 76 |
Ñ15Í21N3ÎS |
72 |
|
|
2.13 |
CH3 |
nonyl |
118 - 120 |
Ñ19Í29N3ÎS |
65 |
|
|
2.14 |
CH3 |
decyl |
63 - 66 |
Ñ20Í31N3ÎS |
59 |
|
|
2.15 |
CH3 |
cyclohexyl |
82 - 84 |
Ñ16Í21N3ÎS |
76 |
|
|
2.16 |
CH3 |
benzyl |
195 - 197 |
Ñ17Í17N3ÎS |
79 |
|
|
2.17 |
ÑÍ3 |
phenetyl |
84 - 86 |
Ñ18Í19N3ÎS |
70 |
|
|
2.18 |
CH3 |
2-nitrophenyl |
157 - 160 |
Ñ16Í14N4Î3S |
78 |
|
|
2.19 |
CH3 |
4-nitrophenyl |
123 - 126 |
Ñ21Í16N4Î3S |
63 |
|
|
2.20 |
CH3 |
2-pyrydyl |
189 - 192 |
Ñ15Í14N4ÎS |
64 |
|
|
2.21 |
CH3 |
2-chynolyl |
199 - 201 |
Ñ19Í16N4ÎS |
67 |
|
|
2.22 |
Ñ6Í5 |
àllyl |
75 - 77 |
Ñ22Í19N3ÎS |
72 |
|
|
2.23 |
Ñ6Í5 |
decyl |
53 - 55 |
Ñ25Í33N3ÎS |
81 |
|
|
2.24 |
Ñ6Í5 |
benzyl |
123 - 125 |
Ñ22Í19N3ÎS |
63 |
|
|
2.25 |
Ñ6Í5 |
phenethyl |
211 - 212 |
Ñ23Í21N3ÎS |
81 |
|
|
2.26 |
Ñ6Í5 |
2-nitrophenyl |
147 - 149 |
Ñ21Í16N4Î3S |
77 |
|
|
2.27 |
Ñ6Í5 |
4-nitrophenyl |
136 |
Ñ21Í16N4Î3S |
64 |
|
|
2.28 |
Ñ6Í5 |
2-pyrydyl |
209 - 211 |
Ñ20Í16N4ÎS |
54 |
|
|
2.29 |
Ñ6Í5 |
2-chynolyl |
176 - 178 |
Ñ24Í18N4ÎS |
75 |
|
Contin. tabl. 2.4
|
Ñompound |
Found, % |
Calculated, % |
||||||||
|
C |
H |
N |
S |
C |
H |
N |
S |
|||
|
1 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
||
|
2.11 |
59,31 |
6,54 |
15,95 |
12,17 |
59,29 |
6,51 |
15,96 |
12,18 |
||
|
2.12 |
61,84 |
7,24 |
14,44 |
11,03 |
61,82 |
7,26 |
14,42 |
11,00 |
||
|
2.13 |
65,64 |
8,39 |
12,07 |
9,25 |
65,67 |
8,41 |
12,09 |
9,23 |
||
|
2.14 |
66,47 |
8,63 |
11,65 |
8,89 |
66,44 |
8,64 |
11,62 |
8,87 |
||
|
2.15 |
63,32 |
6,97 |
13,88 |
10,59 |
63,33 |
6,98 |
13,85 |
10,57 |
||
2.16 |
65,59 |
5,53 |
13,46 |
10,33 |
65,57 |
5,50 |
13,49 |
10,30 |
||
|
2.17 |
66,41 |
5,85 |
12,94 |
9,88 |
66,43 |
5,88 |
12,91 |
9,85 |
||
|
2.18 |
56,10 |
4,15 |
16,33 |
9,34 |
56,13 |
4,12 |
16,36 |
9,37 |
||
|
2.19 |
56,15 |
4,16 |
16,33 |
9,34 |
56,13 |
4,12 |
16,36 |
9,37 |
||
|
2.20 |
60,41 |
4,71 |
18,81 |
10,78 |
60,38 |
4,73 |
18,78 |
10,75 |
||
|
2.21 |
65,53 |
4,60 |
16,11 |
9,21 |
65,50 |
4,63 |
16,08 |
9,20 |
||
|
2.22 |
66,82 |
5,33 |
12,96 |
9,93 |
66,85 |
5,30 |
12,99 |
9,91 |
||
|
2.23 |
70,91 |
7,82 |
9,93 |
7,56 |
70,88 |
7,85 |
9,92 |
7,57 |
||
|
2.24 |
70,73 |
5,16 |
11,23 |
8,57 |
70,75 |
5,13 |
11,25 |
8,59 |
||
|
2.25 |
71,32 |
5,48 |
10,81 |
8,25 |
71,29 |
5,46 |
10,84 |
8,27 |
||
|
2.26 |
62,39 |
4,02 |
13,82 |
7,90 |
62,36 |
3,99 |
13,85 |
7,93 |
||
|
2.27 |
62,38 |
3,96 |
13,88 |
7,91 |
62,36 |
3,99 |
13,85 |
7,93 |
||
|
2.28 |
66,62 |
4,49 |
15,57 |
8,94 |
66,65 |
4,47 |
15,54 |
8,90 |
||
|
2.29 |
70,26 |
5,37 |
18,95 |
10,77 |
70,22 |
4,42 |
13,65 |
7,81 |
||
Table 5
Systems of solvents for thin
layer chromatography of synthesized compounds
|
Solvent’s system |
Solvents |
Correlation of
solvents |
|
1 |
Acetone : hexane |
2:3 |
|
2 |
Åthanole : chloroform
: methanole |
1:1:1 |
|
3 |
Chloroform :
åthylacetate |
2:3 |
|
4 |
Chloroform :
ethylacetate |
3:2 |
|
5 |
Àcetone : hexane :
chloroform |
1:1:1 |
Individuality of compounds 2.11-2.29 confirmed by thin
layer chromatography method in different solvent’s systems (Table. 2.5).
In the IR-spectra of 4-(2-methoxyphenyl)-3-alkyl
(aryl-, heteryltio-)-5-methyl-4H-1,2,4-triazoles and
4-(2-methoxyphenyl)-3-alkyl (aryl-, heteryltio-)-5-phenyl-4H-1,2,4-triazoles
present absorption bands of C=N-groups within 1666-1467 cm-1 and
absorption in 1604-1467 cm-1 area and below 1030 cm-1,
that confirms the aromatic fragment presence.
Planar deformation vibrations of C-H group within
1030-1004 cm-1 and 793-744 cm-1 in its turn prove the
presence of 2-methoxypenyle fragment. Additionally, available vibrational bands
of methoxygroups within 2834-2850 cm-1.
As compared with IR-spectra of initial compounds -
4-(2-methoxyphenyl)-5-methyl-1,2,4-triazole-3-thione (2.9) and
4-(2-methoxyphenyl)-5-phenyl-1,2,4-triazole-3-thione, IR-spectra of their
3-alkylthio-derivatives (2.11-2.29) have absorption bands at 2975-2610 cm-1
and 1250-1175 cm-1, which may show the presence of methyl or
methylene radicals. Within 1710-1650 cm-1 are absent bands that can
be caused by C=S-groups.
Conclusions
Established
the optimal conditions of alkylation,
arylation, heterylation of 4-(2-methoxyphenyl)-5-methyl-1,2,4-triazole-3-thione
(2.9) and 4-(2-methoxy-phenyl)-5-phenyl-1, 2,4-triazoles-3-thione (2.10). It is
proved that the major outputs of the reaction products were observed using
ethanol as a solvent. Researched general physical and chemical properties of
the compounds.
Literature
1. Pat. 2010/0168122 À1US, Int.Cl. C07D
473/04, À61Ê 31/522. Xanthine derivatives as selective
HM74A agonists / R. J. D. Hatley, A. M. Mason, I. L. Pinto. – Çàÿâë. 08.08.2006;
îïóáë. 01.07.2010.
2. Pat.
7560450 B2 US, Int.Cl. C07D 473/06, À61Ê 31/522.
Xanthine derivatives, the preparation thereof and their use as pharmaceutical
compositions / M. Eckhardt, F. Himmelsbach, E. Langkopf, R. Maier. – Çàÿâë. 18.11.2003;
îïóáë. 14.07.2009.
3. Pat. US
2012/0065236 À1, Int. Cl.
A61K 31/426, À61Ð 19/06, A61P
11/00. Methods for concomitant of theophylline and febuxostat / L.
Gunaward-hana, M. Tsai, H. Naik. – Çàÿâë. 08.09.2011; îïóáë. 15.05.2012.
4.
Raafat M. Shaker. The chemistry of mercapto- and thionsubstituted 1,2,4-triazoles and their utility in
heterocyclic synthesis / Raafat M. Shaker // ARKIVOC.
– 2006. – Vol. IX. – P. 59 – 112.
5. The
synthesis and the biological evaluation of new thiazolidine-4-one derivatives
containing a xanthine moety / F. G. Lupascu, O. M. Dragostin, L. Foia, D.
Lupascu et al. // Molecules, 2013. – ¹ 18. – P. 9684 – 9703.
6. Ëåâ³÷ Ñ. Â. Ñèíòåç òà ô³çèêî-õ³ì³÷í³ âëàñòèâîñò³ S-çàì³ùåíèõ ïîõ³äíèõ 3-áåíçèë-8-ìåòèë-7-[(4-ôåí³ë-5-ò³î-4Í-1,2,4-òð³àçîë-3-³ë)ìåòèë]-êñàíòèíó / Ëåâ³÷ Ñ. Â., Øêîäà Î. Ñ., Àëåêñàíäðîâà Ê. Â. // Àêòóàëüí³ ïèòàííÿ ôàðìàöåâòè÷íî¿ ³ ìåäè÷íî¿ íàóêè òà ïðàêòèêè, 2013. – ¹ 1 (11). – Ñ. 54 – 58.
7. Ïàò. 61715, ÌÏÊ (2011.01), Ñ07D
473/00. Àì³ä
4-ôåí³ë-5-(3'-ìåòèëêñàíòèí³ë-7')ìåòèë-1,2,4-òðèàçîë³ë-3-ò³îàöåòàòíî¿ êèñëîòè,
ÿêèé âèÿâëÿº ä³óðåòè÷íó, ïðîòèçàïàëüíó òà àíàëãåòè÷íó 䳿 / Þð÷åíêî Ä. Ì.,
Àëåêñàíäðîâà Ê. Â., Ðîìàíåíêî Ì. ²., Ñàìóðà Á. À., Òàðàí À. Â. – Çàÿâë.
17.01.2011; îïóáë. 25.07.2011.
8. Ñèíòåç, ðåàêö³¿ òà ô³çèêî-õ³ì³÷í³
âëàñòèâîñò³ ïîõ³äíèõ 8-ò³îêñàíòèí³ë-7-àöåòàòíèõ êèñëîò / Ä. Ì. Þð÷åíêî, Ê. Â.
Àëåêñàíäðîâà, Ì. ². Ðîìàíåíêî, Î. Á. Ìàêî¿ä // Àêòóàëüí³ ïèòàííÿ ôàðìàö. ³ ìåä.
íàóêè òà ïðàêòèêè. – 2011. – Âèï. XXIV, ¹ 3. – Ñ. 104 – 108.