Wus K. O., Kutsenko O. K., Yudintsev A. V., Trusova V. M.,
Gorbenko G. P.
V.N. Karazin Kharkiv National University, 4 Svobody Sq.,
Kharkiv, 61077, Ukraine
BINDING OF NEW CYANINE DYE TO
FIBRILLAR AGGREGATES OF LYSOZYME
|
|
|
|
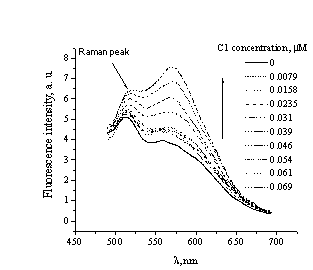
Fig. 1.
Fluorescenñe intensity
spectra of C1 free in solution and bound to
fibrillar lysozyme |
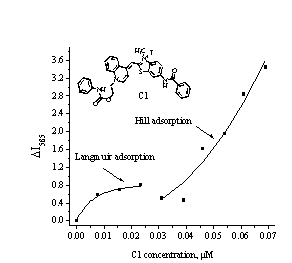
Fig. 2. The
isotherm of C1 binding to fibrillar Lz. Protein concentration was 1.1 µM |
Due to a strong causative link
between protein amyloidogenesis and development of so-called conformational
diseases, such as type II diabetes, spongiform encephalopathies etc., correct detection of protein
pathological aggregates represents one of the acute problems [1, 2]. In this regard, cyanine
dyes, typically used for DNA detection, have very attractive properties: i) large
Stokes shift; ii) high extinction coefficient; iii) very weak fluorescence in
aqueous solution [3]. The aim of the present study was to evaluate the potential of
novel cyanine dye C1 for identification of amyloid fibrils of small cationic
protein lysozyme (Lz).
The
first step of the study involved obtaining the adsorption isotherms by examining
C1 spectral behavior in the absence and presence of fibrillar lysozyme, highly
ordered aggregates with a core cross-β-sheet structure,
in which β-strands run perpendicularly
to the long axis of the fibril [2]. The asymmetric C1 dye was synthesized in the
University of Sofia. Protein fibrils were prepared by dissolving Lz in
deionized water with subsequent slow addition of ethanol to a final
concentration of 80% and constant agitation at ambient temperature. Fluorescence
spectra were obtained during titration of fibrillar Lz solution with C1, using
Perkin Elmer LS-55 spectrofluorimeter.
As
shown in Fig. 1, C1 binding to Lz aggregates resulted in two-fold increase in the
fluorescence intensity. The resulted adsorption isotherm has bimodal character
(Fig. 2) and was analyzed within the framework of two binding models – Langmuir
and Hill [4]. The first one can be written as:
![]() (1)
(1)
where ![]() ,
, ![]() are the total
concentrations of the dye and protein, respectively;
are the total
concentrations of the dye and protein, respectively; ![]() – the number of the dye
binding sites per protein molecule, Ka
is association constant, a – coefficient of proportionality. Presented in Table 1 are thermodynamic parameters of C1-Lz binding
(Langmuir), obtained by fitting the initial part of experimental curve by Eq.(1).
Notably, the recovered stoichiometry was comparable with that reported for Thioflavin
T [2]. The high Ka is indicative of the dye binding to specific
sites of the fibrils. The dye-protein complex is probably stabilized by H-bonds
and hydrophobic interactions of C1 with β-sheets. Taken into
account that the molecular dimensions of C1 (2.3×0.7 nm) are similar to
those of Congo Red (2.6×0.9 nm), we assumed that these dyes have similar
binding sites, i. e. the binding occurs in the grooves of amyloid fibrils [2].
– the number of the dye
binding sites per protein molecule, Ka
is association constant, a – coefficient of proportionality. Presented in Table 1 are thermodynamic parameters of C1-Lz binding
(Langmuir), obtained by fitting the initial part of experimental curve by Eq.(1).
Notably, the recovered stoichiometry was comparable with that reported for Thioflavin
T [2]. The high Ka is indicative of the dye binding to specific
sites of the fibrils. The dye-protein complex is probably stabilized by H-bonds
and hydrophobic interactions of C1 with β-sheets. Taken into
account that the molecular dimensions of C1 (2.3×0.7 nm) are similar to
those of Congo Red (2.6×0.9 nm), we assumed that these dyes have similar
binding sites, i. e. the binding occurs in the grooves of amyloid fibrils [2].
|
Table
1. Quantitative parameters of the dye binding to fibrillar lysozyme |
||||
|
Binding model |
Ka , |
|
|
|
|
Langmuir |
6900 |
0.01 |
– |
|
|
Hill |
8.27 |
1.77 |
2.55 |
|
Next, sigmoidal
part of the adsorption isotherm was analyzed using the Hill binding model. This
model suggests absolute cooperativity of the dye-protein interactions:
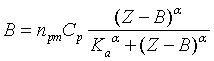 (2)
(2)
where B – concentration of the
bound dye, ![]() is Hill parameter.
Calculation of the binding parameters in terms of this model showed that Ka is 3 order of magnitude lower
than in the case of specific binding (Table 1, Hill). The Hill parameter
is Hill parameter.
Calculation of the binding parameters in terms of this model showed that Ka is 3 order of magnitude lower
than in the case of specific binding (Table 1, Hill). The Hill parameter ![]() is greater than 1, reflecting
the cooperativity of C1-fibril association [4]. This model assumes nonspecific saturation
of sites. There was no saturation observed for adsorption, considered in terms
of Hill binding model (Fig. 2).
is greater than 1, reflecting
the cooperativity of C1-fibril association [4]. This model assumes nonspecific saturation
of sites. There was no saturation observed for adsorption, considered in terms
of Hill binding model (Fig. 2).
At the last step of the study C1
binding to amyloid fibrils was analyzed by titration of the dye solution with
Lz. Fitting the experimental results by Hill model yielded the values of
binding parameters close to those presented in Table 1. The grooves, abundant
in the structure of amyloid fibrils, may serve as potential specific binding sites for C1, while
nonspecific ones may appear as a result of the dye self-association [2].
In summary, the present study revealed
a complex nature of interactions between cyanine dyes and lysozyme fibrils,
which must be taken into account while using this class of fluorophores for
amyloid detection and characterization. This work was supported by the grants
from Science and Technology Center in Ukraine (Project number 4534) and
Fundamental Research State Fund (Project number F28.4/007).
References
1. Volkova K. D., Kovalska V. B., Balanda A. O. Cyanine dye–protein interactions: Looking for fluorescent probes for amyloid structures // J. Biochem. Biophys. Methods. – 2007. – V. 8 – P. 1 – 7.
2. Minna G. Binding mode of Thioflavin T and other
molecular probes in the context of amyloid fibrils – current status // J. Chem.
Biol. – 2010. – V. 3. – P. 1 – 8.
3. Deligiorgiev T. G.,
Zaneva D. A. A novel method for the preparation of monomethine cyanine dyes //
Dyes and Pigments. – 1999. – V. 41 – P. 49 – 54.
4. Cantor C., Shimmel
P., Biological Chemistry. Part III. The behavior of biological macromolecules. –
San Francisco: W. H. Freeman and company, – 1980. – 536 p.