Kastornaya A.P., Yudintsev A.V., Trusova V.M., Gorbenko G.P.
V.N. Karazin Kharkiv National University, 4 Svobody Sq.,
Kharkiv, 61077, Ukraine
MODIFICATION OF MODEL MEMBRANES
UNDER THE INFLUENCE OF OLIGOMERIC LYSOZYME
The correlation between
neurodegenerative diseases (Parkinson’s, Alzheimer’s and Huntigton’s diseases),
type II diabetes, systemic amyloidosis, etc. and amyloid aggregation in
brain tissue has long been established. A growing
body of evidence has demonstrated that amyloid protein-membrane interactions may
underlie the cytotoxic effects elicited by amyloid proteins. A number of recent
studies suggest that amyloid toxicity arises primarily from a soluble
oligomeric form of the peptide rather than amyloid monomers or mature fibrils. Membrane-associated mechanisms of amyloid cytotoxicity
include membrane depolarization, bilayer destabilization, pore or ion channel
formation, and membrane-associated free radical generation [1,2]. However, the membrane
effects of mature fibrils so far are poorly understood. In view of this, the
present study has been undertaken to ascertain the influence of fibrillar
lysozyme on the structure of model membranes (liposomes) composed of phosphatidylcholine
(PC) and its mixture with cardiolipin (CL) (5 and 10 mol%) and cholesterol (30
mol%). To this end, fluorescent probe 6-Lauroyl-2-(N,N-dimethylamino)naphthalene (Laurdan), highly sensitive to the environmental polarity, has been
employed. The structure of Laurdan molecule and its fluorescence
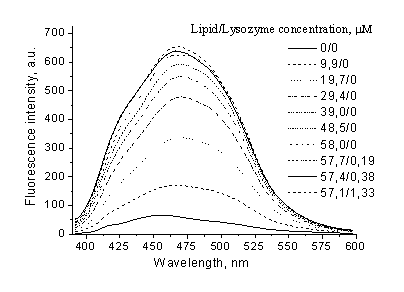
spectra in PC liposomes are shown in Fig.
1. Unilamellar lipid vesicles composed of PC and its
mixtures with CL or cholesterol were prepared by the extrusion method. Amyloid
fibers of lysozyme were obtained by protein incubation in 80% ethanol under
continuous agitation during 30 days.
Quantifying of Laurdan partition coefficient
For quantitative description
of Laurdan binding to liposomes of varying composition the results of fluorimetric
titration were treated in terms of
partition model. The partition coefficient, KP, is defined as
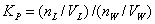 (
(
where nL and nW are
the molar concentrations of the probe in lipid and water phases respectively, VL
and VW are the volumes of respective phases.
|
|
|
|
Fig. 1. Laurdan structure and fluorescence spectra in PC liposomes
|
Based on fluorescence data Kp can be calculated from equation
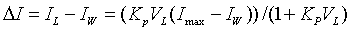 (2)
(2)
where ΔI – fluorescence intensity change , IL, IW – fluorescence intensities in lipid and in water phases,
respectively, Imax – maximum fluorescence intensity of the probe in a
lipid environment [3]. The recovered in such a manner partition
coefficients are presented in Table 1. The results obtained are indicative of
rather high Laurdan affinity for lipid bilayers.
Table
1. Partition coefficients of Laurdan in different lipid systems
|
Liposome composition
|
KP
|
ΔImax
|
|
PC
|
1.4·104±5.4·103
|
1.3·103±3.1·102
|
|
PC:CL (5%)
|
2.1·104±9.7·103
|
5.8·102±1.5·102
|
|
PC:CL (10%)
|
9.2·103±3.2·103
|
7.6·102±1.9·102
|
|
PC:Chol
(30%)
|
5.6·103±3.2·103
|
1.4·102±7.0·102
|
Analysis of Laurdan fluorescence spectra
Laurdan is an amphiphilic
fluorescence probe, whose fluorescence spectra are sensitive to the environmental
polarity (hydration level). In the solvents of high polarity, Laurdan shows
a considerable shift of its emission spectrum to longer wavelengths due to
dipolar relaxation processes. When the local environment of Laurdan is a
phospholipid phase, the emission depends on the packing of the lipid chains. At
temperatures below the phase transition (gel state) the emission maximum is
near 440 nm. At temperatures above the phase transition (liquid crystalline
state) the emission maximum is red-shifted to 490 nm. In the lipid bilayer the Laurdan
molecule is strongly anchored in the hydrophobic core of the bilayer by
hydrophobic interactions between its lauric acid tail and the lipid alkyl tails
while its fluorescing moiety is located at the glycerol level of the
phospholipid headgroups. The changes in the emission spectrum of Laurdan can be characterized by
the generalized polarization value (GP). It has been shown that the GP value decreases when water
penetration into the bilayer increases; this is
due to the foregoing red shift of the Laurdan
fluorescence spectrum caused by dipole–dipole
interactions and reorientation of available
water molecules in the vicinity of the Laurdan probe
in the bilayer [4]. The generalized polarization
was
calculated according to the equation
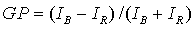 (3)
(3)
where IB and IR are the maximum fluorescence intensities of
the blue and red spectral components, respectively. To obtain the value of this
parameter, fluorescence intensities at
440 (IB) and 490 (IR) nm were used.
|
|
|
|
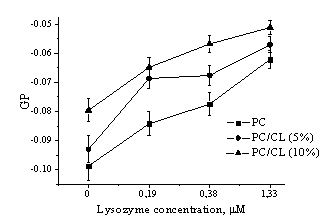
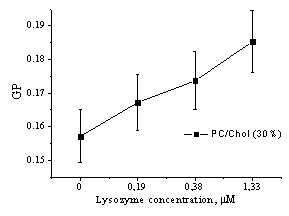
Fig.
2. Generalized fluorescence polarization (GP) of Laurdan lipid probe emission
in vesicles of different composition as a function of amyloid lyzozyme
concentration
|
Excitation wavelength was 364 nm. The GP of
Laurdan in different lipid vesicles as a function of amyloid protein
concentration is shown in Fig. 2. As evident from represented data, the GP was negative (about -0.08 –
-0.1) in liposomes composed of phosphatidylcholine and its mixture with cardiolipin, while it turned
out to attain positive values in the vesicles from PC mixture with cholesterol.
This effect could be explained by condensing influence of cholesterol on the lipid
bilayer. In all types of liposomes increase of fibrillar lysozyme concentration
resulted in the increment of the generalized polarization value. These
findings reveal that amyloid fiblills cause decrease of polarity and increase of lipid packing
density in the model membranes.
To summarize, the present study
provides evidence for modifying effect
of mature lysozyme fibrils on the structure of model membranes. Regardless of
the membrane composition, fibrillar aggregates of lysozyme brought about
reduction of bilayer polarity originated presumably from the increment of lipid
packing density. The most
pronounced polarity decrease (GP increase by ~ 40 %) were observed for PC/CL liposomes,
while in PC/Chol bilayer lysozyme fibrils produced weaker polarity changes (GP
increase by ~ 18 %).
This work was
supported in part by the grant #4534 from the Science and Technology Center in
Ukraine and Fundamental Research State Fund (project number F.28.4/007).
References
1. Valincius G., Heinrich
F., Budvytyte R., Vanderah D.J., McGillivray D.J., Sokolov Y., Hall J.E.,
Losche M. Soluble amyloid
β-oligomers affect dielectric membrane properties by bilayer insertion and
domain formation: implications for cell toxicity // Biophys. J. – 2008. – Vol.
95. – P. 4845 – 4861.
2. Wang S. S.-S., Liu K.-N. Membrane dipole
potential of interaction between amyloid protein and phospholipid membranes is
dependent on protein aggregation state // J. Chin. Inst. Chem. Eng. – 2008. –
Vol. 39. – P. 321 – 328.
3. Santos N.C., Prieto M., Castanho A.R.B.
Quantifying molecular partition into model systems of biomembranes: an emphasis
on optical spectroscopic methods // Biochim. Biophys. Acta. – 2003. – Vol.
1612.– P. 123 – 135.
4. Mukherjee S.,
Chattopadhyay A. Monitoring the organization and dynamics of bovine hippocompal
membranes utilizing Laurdan generalized polarization // Biochim. Biophys. Acta.
– 2005. – Vol. 1714. – P. 43 – 55.
![]() (2)
(2)


