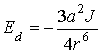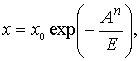Engeneering sciences/ 10 Mining
Akhmatnurov D.R., Akhmatnurov R.R., Musin R.A., Mayer E.A., Sadykov A.S.
Karaganda State Technical University, Kazakhstan
The
sorption relationship of methane and coal
The coal seams the main quantity of gas is physically
or chemically bound state preferably as a solution in a solid (absorption) in
condensed form on the pore surface (adsorption) and condensed in supramolecular
pores micropores with very small their volume (comparable to the volume of
methane molecule) is an area of influence of the sorption forces. These
micropores and gas in the intermolecular space of coal substance (solid
solution of Coal and Gas) determine the basic amounts of methane in coal seams.
Modern ideas about the connection of
methane with coal based on theoretical concepts and experimental data of
physical chemistry of artificial sorbents. Sorption of methane in coal is due
to the presence of micropores. Now to the micropores relate the void size of
not more than 3 nm. In addition to the micropores, in fossil coals distinguish
transient pores ranging in size from 3 to 300 nm and macropores having a size
of more than 300 nm. It is considered that the micropores and transitional
pores partially constitute the main reservoir of gas are adsorbed and sorbed
amounts.
The
adsorbed methane is held dynamically by the coal dispersion forces that occur
between nonpolar molecules. Dispersion forces determine the physical nature of
the connection of methane with coal. This bond makes possible the reversibility
of the sorption processes, i.e. the full correspondence between the amount of
gas adsorbed and desorbed under identical thermodynamic conditions.
To
determine the nature of the relationship of methane with coal was adopted by
the following condition: if the relationship is of a physical nature, the
relationship between the energy of the dispersion interaction of the sorbate
molecules and the magnitude of sorption on coal must be linear.
The
value of sorption of methane on coal (dm3/kg) at temperature T=288 oK
and pressure of 0,1 MPa depends on the energy dispersion relation E in the form:
|
|
(1.1) |
where
![]() – is the mole fraction of gas sorption per
unit of quantity in the free state at a temperature of 288o K and
pressure 1 Pa (
– is the mole fraction of gas sorption per
unit of quantity in the free state at a temperature of 288o K and
pressure 1 Pa (![]() ); Åd.e – critical value
of the dispersion energy of the sorbate, below which the sorption of gas on the
sorbent does not occur; for the case of the analyzed
Åd.k.=0.5 J/mol; Åd – is the energy dispersion relation sorbate,
defined by the formula:
); Åd.e – critical value
of the dispersion energy of the sorbate, below which the sorption of gas on the
sorbent does not occur; for the case of the analyzed
Åd.k.=0.5 J/mol; Åd – is the energy dispersion relation sorbate,
defined by the formula:
|
|
(1.2) |
where
a – is the polarizability of the
sorbate, m3; J – is the
gas ionization potential, J/mol; r –
is the distance from the center of the molecule, m.
As
can be seen from (1.2), with increasing distance between the molecules the
dispersion interaction energy decreases inversely proportional to the sixth
power of the distance. Therefore, dispersion forces are short range and Fd is
practically cease to exist at a distance of 1-2 nm from the surface of the
sorbent. Such distances and the dimensions of the micropores which, as
mentioned, along with a partially transient pores are the main reservoir of
sorbed gas, suggesting that the system methane-coal is, in fact, solid
solution, where the gas in the pores of coal represents the condensate. So now
for the interpretation of sorption isotherms on coal seems more reasonable to
use the developed school M.M. Dubinin Theory of volume filling, which comes
from the knowledge about the porosity of sorbent. To describe sorption of
methane on coal the theory of volume filling used M.F. Yanovsky, I.L. Ettinger,
N.In. Shulman and others. The Main equation of adsorption in the theory of
volume filling is:
|
|
(1.3) |
where
x – is the adsorption under these
conditions, dm3/kg; x0 –
adsorption completely filled with the sorption volume, dm3/kg; A – is the differential molar work of
adsorption, J/mol; E –is the
characteristic energy of adsorption is equal to the molar differential work of
adsorption isotherms at the point where the filling of the sorption volume of
368%, J/mol; n – coefficient
characterizing the pore size of the sorbent (mineral coal n=3-4).
An
important constant in the theory of volume filling of micropore volume is.
According to the data of some coals of Donbass this value varies from 0,03 to
0,09 dm3/kg. For the transition of gas molecules from the free phase into the adsorbed
state, you need to meet the conditions:
|
|
(1.4) |
where
Åd is the energy of
dispersion interaction of one mole of gas, J/mol; R=8,31 J/(mol oÊ) is the gas constant. Obviously:
|
|
(1.5) |
where
U1 and U2 are respectively the
contributions of the molecules of gas and coal in the energy dispersion
relation, J/mol.
Using
(1.3) and (1.5), the condition (1.4) for one mole of gas can be expressed as:
|
|
(1.6) |
The
equal sign determines the state of dynamic equilibrium, the sign of inequality
– the process of sorption of gas absorption.
From
(1.5) it follows an important rule: depending on the molecular structure of the
coal gas in the state of sorption equilibrium has a different dispersion energy
of attraction, which, in particular, specifies that each sorbate in the
sorption takes the volume of its distinctive importance on energy, localized
area.
References:
1. Krivitskaya
R.M., Ettinger I.L., Lidin K.L. i dr. Molekulyarno-sitovoy effekt pri sorbtsii
gazov iskopayemymi uglyami // Khimiya tverdogo topliva. - 1973. - ¹2. - S.
152-154.
2. Biryukov
YU.M., Pimenov A.A., Khodzhayev R.R. Nauchnyye osnovy tekhnogennoy
gazodinamicheskoy bezopasnosti vedeniya gornykh rabot v uglenosnoy tolshche. -
Kaliningrad: FGOU VPO «KGTU», 2009. - 316 s.