DEVELOPMENT
OF THE AUDITORY ORGAN IN
ECHOLOCATING SPECIES
G.N. Solntseva
A.N. Severtsov Institute of Problem Ecology and
Evolution RAS
Leninsky Prospekt, 33, 119071 Moscow, Russia
e-mail: g-solntseva@yandex.ru
Basic features of structure and development of the peripheral auditory
system in different mammalian species are common. Nevertheless, the usage of
only definite acoustic characteristics of habitat by the separate groups of
animals has caused the pronounced polymorphism of all parts of the auditory
system beginning from the outer ear. Development of different habitats by
mammals, forming of various forms of spatial orientation and communication have
been accompanied by substantial morphological transformations of all units of
the peripheral auditory system, especially of the phylogenetically young units,
which are typical only for the mammalian class. During the adaptive radiation
of biological forms, which are phylogenetically distant, but similar in habitat
conditions, similar morphological features in the structure of different organs
appear.
Having studied the features of structural organization
of the outer, middle and inner ears in representatives of echolocating mammals
in postnatal ontogenesis, we found it necessary to carry out a
comparative-embryological research of all parts of the peripheral auditory
system in prenatal ontogenesis (Solntseva,
1992).
The research allowed not only to study structural
features of the auditory organ of investigated species in more detail, but also
to find out the peculiarities of its formation linked to acoustic properties of
their habitat, as well as to find an explanation of the appearance of some
structural adaptations in semi-aquatic and aquatic forms, having defined a
stage of formation.
The study of marine mammals' auditory organ (cetaceans), representing an
absolutely specific direction of placental animals' evolution, was started more
than three centuries ago. However, these works were carried out mainly at an
anatomic level and had a fragmentary character (Reysenbach de Haan, 1957;
Fraser, Purves, 1960; Fleischer, 1973). Besides, the research data on
development of the cetaceans' auditory organ during an early pre-fetal period
were completely absent (according to Schmidt's
periodization, 1968), i.e. beginning with formation
of an acoustic vesicle, or the stage of bud, up to the end of formation of
basic anatomic structures of the auditory organ.
Great difficulties in gathering of embryonic material on marine mammals inevitably led to the fact that organs of hearing and equilibrium in a great group of mammals remained unstudied for a long time and dropped out of the general scheme of study of these organs’ development in mammals as a whole. All this prevented the solution of many questions concerning structural organization of the peripheral auditory system in various groups of mammals and did not allow for determination of the general patterns of the peripheral auditory system's development in mammals as a whole.
The most important data concerning adaptive and evolutionary
changes of the auditory system could be obtained only by comparative studies of
embryogenesis of the
system in a wide set of species, phylogenetically close, but with different
ecologies, as well as in species, phylogenetically distant, but with a similar
way of life.
For the first time we carried out a
comparative-embryological research of the peripheral part of the auditory system
using unique embryonic collections on cetaceans, what allowed for study of
structural organization of this part in more detail, to reveal the features of
similarity and distinction in formation of hearing and equilibrium organs for
different stages of development, and also to determine the stages of formation
of structural adaptations revealed by us earlier.
On the basis of obtained results developmental
patterns of the structures of the outer, middle and inner ears in
representatives of echolocating mammals belonging to various ecological groups
were established.
As it was known, while studying the development of
various organs, including hearing and equilibrium organs, many researchers used
basically a method of comparison the developing structures with the length of
embryos. Such approach was not correct in comparative analysis of prenatal
development of the auditory organ in a wide set of species as their terms of
pregnancy and the length of embryos for similar stages of embryogenesis were
sharply different. However, in experimental embryology conceptions about equivalent stages of development (Otis,
Brent, 1954) were widely known.
To have an opportunity to compare rudiments of
different species, a special research for normal development was undertaken on
some laboratory animals by a group of scientists; as a result, the stages of
development with common features for different species were marked out (Dyban
et al., 1975).
With the view to carry out an adequate comparison on forming the peripheral part of the auditory system in various mammalian species, we applied a principle to compare the developing structures of the outer, middle and inner ear at similar stages of development using known tables on normal development of laboratory animals (Dyban, et al., 1975). In addition to this, the stages of structural formation of the outer, middle and inner ear were compared by us with the stages of mesenchymal tissue replacement by an embryonic cartilage.
As in majority of mammals, a similarity in a sequence of development of the structures of the
outer, middle and inner ears was revealed; for the convenience of the
description we used the known developmental stages of some terrestrial species
(Dyban, et al., 1975).
A pair rudiment of a membranaceous labyrinth is marked
at the stage of 2-3 pairs of somites (Wilson, 1914). At the stage of 6-9 pairs of somites, the membranaceous labyrinth's bud represents an
acoustic placode (Kappers, 1941). Further, at the stage of 14-15 pairs of somites, an acoustic pit is formed, from which an acoustic
vesicle develops at the stage of 20 pairs of somites (13th stage of development), which passes into an endolymphatic
duct without any special borders. At this
stage of development, an auditory ossicles’ bud in the form of mesenchyme’s thickening appears.
In all investigated species a subdivision of the acoustic vesicle into superior and anterior parts occurs at stages
14-15. Both parts are surrounded by mesenchyme. Further, a vestibular apparatus
is formed from the superior part, and a formation of the cochlear canal begins
from the anterior part, in which the basis, composed by a columnar epithelium,
and the roof, consisting of a cuboidal epithelium, are well-distinct (stage 16). In the superior part of the acoustic
vesicle, a differentiation of vestibular part into semicircular ducts and sacks is observed. In a cochlear part, only a lengthened canal is marked out: it is an endolymphatic
duct, from which a cochlear will be formed
later. At this stage, a subdivision of an acoustical nerve into the vestibular
and cochlear branches,
and their ganglions is
observed .
The outer ear of a greater horseshoe bat includes an auricle (pinna) and an external auditory
meatus. The pinna is noted for its huge size and, in contrast to other species
of bats, lacks a tragus. The auditory meatus has a flared shape.
As well as in other terrestrial species, a development
of an auricle begins at the 16th stage in the form of small prominences,
located on the edge of a deepening formed due to a widening of the first
branchial cleft. At the 17th stage, a fusion of these prominences
takes place and an uniform mesenchymal bud of
the auricle is formed. To the beginning of the 18th stage, the auricle acquires
clearer contours. Further, by the end of the 19th stage, an external auditory
meatus starts to fill up by the epithelium cells. This process finishes by the 21th
stage of development (Solntseva, 1999 b).
In studied species, by the beginning of the 20th
stage, the auditory meatus is completely filled with epithelial cells. In
mature-born species, these cells are resorbed by the moment of birth, in
immature-born species, the process of resorption finishes only in an early
postnatal development.
To the middle ear of a greater horseshoe bat, the
following features are typical: the presence of a small-sized tympanic membrane
and its vertical disposition; the reduction of the auditory ossicles' size,
their thinning and the
presence of deep hollows in them; the malleus and incus are joined with each
other at sharp angles in the area of an incudomalleal articulation; the stapes is very compact, its cruses
are thickened and form a small inter-crura aperture; the long process of the
malleus knits
with a wall of the tympanic bone; the size of muscles of the middle ear are
considerably increased. These features in the structure of the middle ear
provide transmitting of ultrasound signals.
As well as in other mammals, the bud of the auditory ossicles appears at the 13th stage of development in the form
of a mesenchyme's thickening. At the 16th stage, the contours of the auditory
ossicles become
apparent, and at the 17th stage, each bud of the auditory ossicles represents an independent formation. Their basis is
formed by immature precartilaginous tissue. At the 18th stage, the elements of
the auditory ossicles are formed. The basis of the auditory ossicles is
formed by mature precartilaginous tissue. But, by the end of the given stage,
the mature precartilaginous tissue has been already replaced by embryonic
cartilage.
Tympanum is formed at the 16th stage in the form of a
narrow channel located below the auditory ossicles' bud. At the 18th stage, tympanic and periotic bones are formed,
and at the 19th stage, a turning of the tympanum around the sagittal and
frontal axes of a body of a prefetus occurs.
Formation of the features connected with the
interposition of the auditory ossicles in a tympanum occurs at the stages
18-19.
The bud of the tympanic membrane is formed at the 16th
stage, and by the 17th stage, it is thick and friable. Its significant thinning
is marked at the 18th stage; the tympanic membrane acquires a three-layer
structure and lays almost vertically on the lateral surface of the middle ear's cavity.
The cochlea of a greater horseshoe bat is huge; it is formed by 3.5 turns. In the cochlea's structure the certain
features aimed at the perception of high-frequency signals are marked. A
significant development is reached by a lower, or basal, turn of the cochlea,
as it is directly connected with the perception of high frequencies.
For the first time Griffin (1958) has noticed, that in
bats the round window contacts with the liquid of the inner ear in a quite
another place, than it does in other mammals, i.e. not at the end of the
cochlea, but almost at a millimeter further than its first turn. Later on, he
has revealed that the basilar membrane in bats is supplied with two additional
thickenings in that part of the cochlea which is connected with the perception
of the signals, having the important biological significance for these animals.
The basilar membrane is narrow and thin and is very rigidly fixed between the primary
and secondary osseous spiral laminas.
At the 13th stage of development, the acoustic vesicle
is formed, as well
as in other mammals, which on the stages 14-15 is subdivided into two parts. The vestibular apparatus
is formed from the superior part, and the cochlear canal is formed from the
inferior part.
At the 16th stage, the cochlear canal starts to twist spirally and the
basal cochlea's turn is formed. At the 17th stage, the medial turn is formed,
and at the 18th stage – the apical one and a half-turn. By the end of the 18th
stage the cochlea is anatomically formed with 3.5 turns.
Further at the 19th stage, a formation of the elements
of the cochlea's canal and differentiation of the cells of Corti's organ's
occur, beginning from the basal turn of the cochlea and being gradually extended
to the turns located above. Therefore, in all turns of the cochlea a different
degree of anatomic formation of the cochlear canal and the cytodifferentiation
of Corti's organ is noticed. Among the structures of the cochlear canal a
Reissner’s membrane is the earliest to form, and a vascular stria is the latest
(Fig. 1).
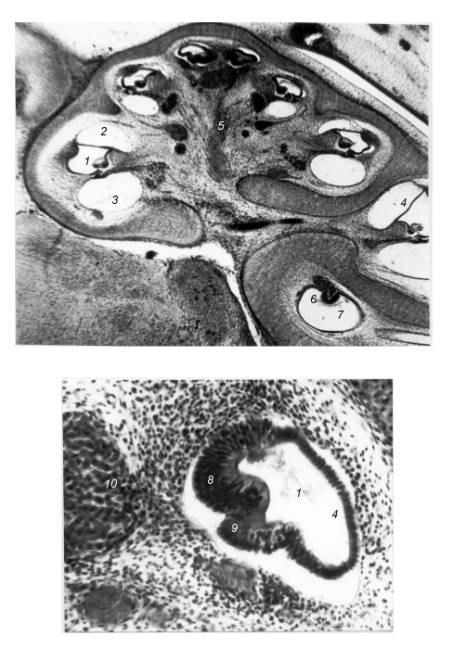
Fig. 1. The cochlea of Rhinolophus ferrumequinum’s prefetus, stages 20 –
21. 1 – cochlear canal; 2 -vestibular
scala; 3 – tympanic scala; 4 – Reissner’s membrane; 5 – cochlear branch of n.
acousticus; 6 – crista ampullaris; 7 – ampula of semicircular canal; 8 – axial
thickening; 9 – lateral thickening; 10 - spiral ganglion
Thus, the development of the auditory organ of a
greater horseshoe bat reveals a great similarity with terrestrial mammals. All
structures of the outer, middle and inner ear are formed from homologous rudiments, in the certain sequence and at the similar
stages of development.
Cetaceans represent one
of two orders of recent mammals, who have completely adapted to aquatic way of
life. However, alongside with general morphological features, typical to all
representatives of the order, in each of the suborders species-specific
features in the structure of organs and systems have appeared. Mysticetes have
kept the olfactory analyzer and have not acquired an ability to echolocation,
but odontocetes, having lost the olfactory analyzer, have got amazing
opportunities for echolocation.
Well-developed hearing of odontocetes is supplemented
with special organs of sound signals' generation. The combination of perfect
auditory receiver with organs of the sound signals’ production has provided the
odontocetes with unlimited opportunities for orientation in water environment
by a reflected echo signal.
The stenella's
and beluga's outer ear, as well as in other representatives of cetaceans,
includes the external auditory meatus only, as the auricle is completely
reduced.
At the 16th stage, from the lateral walls of the first
branchial cleft the cartilaginous part of the external auditory meatus starts
to develop in the form of a short and slightly bent tube opened along the whole
extent. The osseous part of the auditory meatus is forming later. Filling of
the cartilaginous part of the auditory meatus with epithelial cells occurs at
the 19th stage, and its total closing by these cells comes to an end by the
21st stage of development (Solntseva,
1983, 1999 a).
At the 19th stage, the formation of the
species-specific features of the outer ear in the form of a noticeable
narrowing of the auditory meatus in its distal part is marked. Further, the
auditory meatus lengthens and acquires a double bend shape, typical for
definitive forms. To the 20th stage, the auditory meatus widens in the proximal
part. At the later stages, the increase in the absolute size of the auditory
meatus, which is carried out proportionally to the growth of a prefetus, is
marked.
For the first time, we have found out the source of
the epithelioid obliteration in the distal part of the auditory meatus of some
dolphins and a white whale (Solntseva, 1992, 1999 a). In odontocetes, the
structure of the auditory meatus differs from such of all investigated species
of mammals. Only in odontocetes, the auditory meatus has a strongly pronounced
S-shaped form. At some distance from the lumen, the cavity of the auditory
meatus obliterates, as a result, its two parts are formed, distal and proximal
ones. As we have already mentioned, at the 20 stage, the auditory meatus is
already completely filled with epithelial cells, which in immature-born species
are resorbed completely in the early postnatal ontogenesis only, and in
mature-born - by the moment of birth. In mature-born odontocetes, the complete
resorption of epithelial cells occurs in the proximal part of the auditory
meatus only, while in the distal part, a piece of the embryonic epithelial
obliteration is left, not being exposed to a resorption, and later the
epithelial tissue of adult forms, which has been found out by us earlier, is formed
upon its basis (Solntseva, 1971).
All elements of the middle ear develop from
mesenchymal and mesodermal elements. In a spotted dolphin and in a white whale, as well as in other species of
mammals, the bud of the auditory ossicles appears at the 13th stage in the form
of a mesenchymal condensation. At the 14-15th stages, the contours of the auditory ossicles’ buds
are visible, but their form is not similar to that they will adopt at the later
stages of development. Junction between the auditory ossicles is continuous.
The differentiation of the auditory ossicles into the elements, forming them,
is absent. In the mesenchyme, from which the buds of the auditory ossicles
consist, large nuclei, occupying the most part of a cell, are found.
In the bud at the 16th stage, the contours of the
auditory ossicles are revealed and the process of the tympanum’s formation
starts. The buds of the auditory ossicles slowly plunge into the depth of the
tympanum. The basis of the auditory ossicles is formed by a mature precartilaginous
tissue, the cells of which acquire more distinct outlines. At the given stage,
the process of differentiation of the precartilaginous tissue to the embryonic
hyaline cartilage begins. The process of cartilaginification begins in the center of each bud of the auditory
ossicles and spreads gradually to their periphery. Around the chondrocytes the
pericellular substance
is located. Cells’ nuclei are large and surrounded by a narrow band of cytoplasm and are located at a distance from each
other. The auditory ossicles are surrounded by a perichondrium, which consists
of small flat cells, whose chondroblasts have no distinct borders. Due to the
perichondrium the areas of the auditory ossicles junctures are clearly visible.
At the given stage of development, the differentiation of the tympanic
membrane-ligament, stapedius and tympanic muscles starts. The tympanum is
represented by a narrow blind canal located below the buds of the auditory
ossicles.
At the 17-18th stages of development, there is a formation of
tympanic and periotic bones. At the 19th stage, the tympanum's turning around
the sagittal and frontal axes of a body of an animal occurs. The location of
the auditory
ossicles in the tympanum is similar to definitive forms, i.e. the malleus and incus
are connected with each other at right angles.
At the 18th stage, the formation of structural
elements of the auditory ossicles is marked. The auditory ossicles are
increased in size. In the malleus, a head, a neck and a handle are
well-expressed. In the incus, a body and both processes are formed. In the
stapes, there is no differentiation into cruses; therefore the stapes acquires
the form of a smoothed cone. The basis of the auditory ossicles is formed by a
mature precartilaginous tissue. Further, the replacement of the mature
precartilaginous tissue by the embryonic hyaline cartilage occurs. The process
of cartilaginification of the auditory ossicles starts in the center of each bud and
spreads gradually to their periphery.
The formation of the features connected with interposition of the auditory
ossicles in the tympanum of a spotted dolphin and a white whale is marked at
the 16th stage of development, whereas in the majority of the species, which
don't possess abilities for echolocation, it occurs on the 18-19th stages of development.
The tympanic membrane is formed in the
place of the contact of the entoderm of a pharyngeal recess and the ectoderm of the first branchial cleft. The bud of the tympanic membrane appears at the 16th stage and to the 17th
stage it is thick and friable. At the 18th stage, the tympanic membrane becomes
considerably thinner, acquires a three-layer structure and is located almost
horizontally on the lateral surface of the middle ear's cavity. The ligament,
connecting the tympanic membrane with the handle of the malleus, is formed. In
odontocetes and
mysticetes the tympanic membranes reveal similarity in structure at similar
stages of development, whereas during the fetal period they adopt
species-specific features.
The
formation of a cavernous plexus is marked at the 18-19th stages. The development of the venous and peribullar
sinuses occurs a little bit later, beginning from the 21st stage of
development. The replacement of the cartilaginous tissue by the osseous tissue
is marked at the 20th stage in the form of separate centers of ossification in
the integumentary bones of a cranium, tympanic and periotic bones. The initial
ossification of the auditory ossicles is marked at the 21st stage. The process
of formation of the ear muscles and a ring-shaped ligament of the stapes has
ended. The aural capsule is formed by a slightly differentiated cartilage.
In the inner ear at the 13th stage, the acoustic
vesicle develops; its subdivision into superior and inferior parts occurs at the
15th stage like in other mammals. The vestibular apparatus is formed out of the
superior part, and from inferior part the cochlear canal is formed.
At the 16th stage, the cochlear canal
starts to twist spirally, forming a lower, or a basal turn of the cochlea,
which is surrounded by the aural capsule consisting of a compact mesenchyme.
Cellular elements of Corti's organ are approximately on identical stage of
differentiation.
At the 17th stage, the next turn of the cochlea is formed. The
process of formation of turns is accompanied by the formation of a cochlear
nerve (n. cochlearis). The dorso-medial
part of this nerve goes to the apical
turn, and its ventro-lateral one - to the basal turn, the size of which considerably surpasses those of
the turn located above.
At the 18th stage, the cartilaginification of an aural
capsule begins. The cochlea is formed by 2.0 turns. The beginning of the
cellular differentiation of Corti's organ is marked. At this stage, the
columnar epithelium of the cochlear canal moves apart, therefore two
thickenings are formed: an axial and a lateral, from which the elements of the
cochlear canal and Corti's organ are formed. Nuclei of the cells of the future
Corti's organ are large, have an oval form and include numerous nucleoli. Cells
have not formed a typical mosaic in their location yet. Their cytoplasm is
light and hardly visible. The structure and location of these cells enable to
suppose, that further the outer hair cells will be formed from them. Under
these cells the cells with large nuclei including nucleoli are located. Most
likely, it is Deuter's cells; under them the cells with small pyknotic
nuclei are located. The cytoplasm of these
cells is light and forms a narrow band. These are epithelial cells under which
the basilar membrane is located. The Hensen's and Claudius's cells are
presented by a cuboidal epithelium located in 1-2 rows. The Reissner’s membrane
is formed. The formation of a cavernous plexus is marked (Fig. 2).
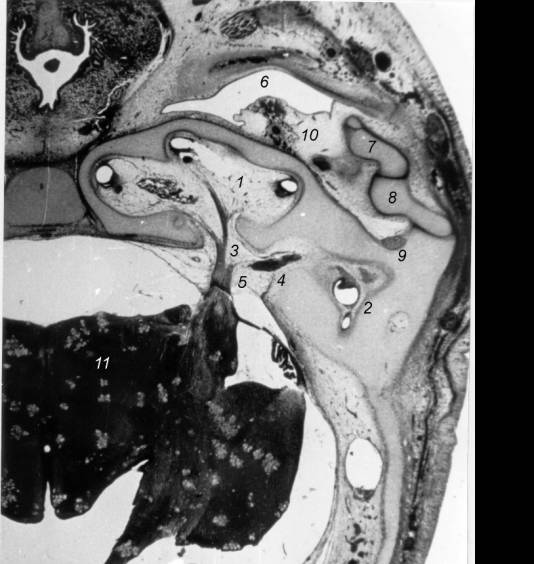
Fig. 2. Histotopography of the peripheral auditory system in
dorsoventral head sections in Stenella attenuata’s prefetus, stages 18-19. 1 – cochlear canal; 2 – vestibular
apparatus; 3 –cochlear branch of n. acousticus; 4 – vestibular branch of n.
acousticus; 5 – n. acousticus; 6 – tympanum; 7 – malleus; 8 – incus; 9 – m.
stapedius; 10 - cavernous plexus; 11 – cerebrum.
At the 19th stage, the supporting
cells, as well as the receptor cells of Corti’s organ, are involved in the
process of differentiation. Cells are located in the order, forming 3 rows. The
nuclei of the cells have a roundish and an oval form. The outlines of the cells
are clearly visible. The neurons of the spiral ganglion are large and densely
adjoin each other, forming characteristic clusters. The formed modiolus is
penetrated by numerous blood vessels.
At the 20th stage, the formation of
the supporting elements of Corti's organ (outer cells-columns) continues. These
cells have a thin bent body with a nucleus located in the basal part of a cell.
Cells of a cylindrical form with a roundish basis are differentiated
simultaneously. These are the internal hair cells. Cells of the
precartilaginous tissue acquire more distinct borders, and in the central sites
they increase in size. The quantity of the intercellular substance grows. The
cochlea is increased. In the cochlear canal the differentiation of a spiral
limb, a vascular stria and a spiral incisure began. The spiral limb is
represented by the cells of an extended form. The vascular stria is formed by
the undifferentiated epithelium. The future spiral incisure consists of a
multi-row high columnar epithelium including transparent nuclei of an oval and
roundish form. The nucleus includes one nucleolus which is centrally located.
The outlines of the cells are hardly visible. The basal membrane, on which the
cells of Corti's organ are located, consists of a connective tissue. The tunnel
is not formed yet. The sizes of the neurons of the spiral ganglion are
increased and nerve fibres are clearly visible. Their nuclei are large, have a roundish
form and are eccentrically located.
At the 21st stage, the connection of the tympanic
membrane-ligament with the handle of the malleus is clearly visible. The
process of formation of ear muscles and a ring-shaped ligament of the stapes
has finished. The aural capsule is formed by a slightly differentiated
cartilage. In comparison with the previous stage, the cochlea acquires much
bigger sizes. The basic process of the cellular differentiation of Corti's
organ has ended. In the cartilage there is an extension of the intercellular
substance and the capsules of the cartilaginous cells are clearly visible. The
process of formation of the isogenic groups of chondrocytes occurs. In the cochlear canal
the process of formation of the tunnel begins. The cells of the spiral ganglion
are located more rarefiedly.
In the white whale’s embryo of the length of 250 mm
the auditory ossicles are connected at right angles in the area of the
incudomalleal joint. In
the malleus the centers of ossification have appeared. The tympanic membrane is
thick; it is connected with the handle of the malleus with the help of a
ligament. The long process of the malleus knits with the wall of tympanic bone.
The structures of the cochlear canal are basically formed. The tympanic and vestibular
scalae, the cochlear canal, the Reissner’s membrane and the spiral ligament are
distinctly visible; the differentiation of the cells of Corti's organ's
continues.
In the process of embryogenesis of the peripheral part
of the stenella's and beluga's acoustic analyzer the morphological
differentiation and maturation of the structures of the outer, middle and inner
ear occurs in the same sequence, as in echolocating bats (Solntseva, 1983, 1999
a).
Thus, in the early embryogenesis of echolocating species the original features of the auditory organ's
formation are found, which are connected with the way of life as well as with
the perception of frequencies of a wide range. Adaptive features in the
structure of the auditory organ are revealed at different stages of
embryogenesis, even at the earliest, in spite of the fact, that the development
in the mother’s womb occurs without direct influence of the environmental
conditions.
The results of comparative study of the peripheral auditory
system's development in representatives of echolocating species shown that
formation of their structures of the outer, middle and inner ear in the early
prefetal period occurs at a similar sequence and approximately at the similar
stages of development. The greatest similarity in the formation of the
peripheral auditory system of mammals is marked in the first half of the early
prefetal period. Species-specific features in the structural organization of
the auditory organ are formed in the second half of the early prefetal period,
depending on an ecological specialization of species. The process of cellular
differentiation of Corti's organ and resorption of epithelial cells of the
auditory meatus in the mature-born animals (cetaceans) finishes, basically, by
a moment of their birth (Solntseva, 2007).
In the immature-born species (bats), the differentiation of elements of the cochlear canal,
cells of Corti's organ, and also the resorption of the epithelium of the
auditory meatus comes to the end only by the 25-30th days (Airapetyantz,
Konstantinov, 1974), since a part of their fetal period is completed only after
the birth.
In echolocating forms (bats, dolphins), belonging to different
taxonomic and ecological groups, the development of middle and inner ears
acquired general properties due to the parallel evolution during which the
development of traits for their intraspecific acoustic communication have been
created in conditions, adverse for vision, and in connection with specific
properties of the environment as a chanal of acoustic communication.
On the basis of results obtained, the following
general regularities of the peripheral auditory system’s development in
representatives of different ecological groups have been determined:
1. In first half of an early prefetal period (stages
13-16), the peripheral auditory system has common features in structure in most
of mammals;
2. Species-specific features in the structural
organization of the peripheral auditory system are formed in the second half of
an early prefetal period (stages 18-20), depending on an ecological
specialization of species;
3. The morphological features of the mammalian
peripheral auditory system, which were formed in the early prefetal period,
continue to develop in the late prefetal, fetal periods, and during an early
postnatal ontogenesis.
REFERENCES
Airapetiantz E.Sh.,
Konstantinov A.I. 1974.
Echolocation in Nature. M.: Nauka,
205-208 (In Russian).
Dyban
A.P., Puchkov V.F., Baranov V.S. etc. 1975. The laboratory mammals: a mouse
Mus musculus,
a rat Rattus norvegicus, a rabbit Oryctolagus cuniculus, a hamster
Cricetus
griseous. In: Subjects of Developmental Biology. M.: Nauka, 505 – 563
(In Russian).
Fleischer G. 1973a. On
structure and function of the middle ear in the bottlenosed dolphin (Tursiops truncatus). Proc. 9th
Ann. Conf. Biol. Sonar and Diving Mammals. Standford Research Institute Press, pp.137
- 179.
Fraser F. Ñ., Ðurves. P. E. 1960. Hearing in Cetaceans. Bul.
Brit. Mus. Natur. Hist. Zool., 7 ( 1 ):
1-140.
Griffin D.R. 1958. Listening
in the dark. The acoustic orientation of bats and men. Yale Univ. Press, New
Haven, p. 413.
Kappers A. 1941. Kopfplacoden
bei Wirbeltieren. Ergebn. Anat. und Entwicklungsgeshichte, 33: 370.
Otis E.M.,
Brent R. 1954. Equivalent ages in
mouse and human embryos. Anat. Rec., 120: 33 - 63
Reysenbach de Haan F. W. 1957a. De gehoorzin van Cetacea.
Vakbl. biol., 37 ( 8 ): 117-127.
Solntseva G.N. 1971.
Comparative-anatomical and histological peculiarities of the outer
and inner ear structures of some
dolphins. In: Marine mammals’ research.
Kaliningrad, 22: 237 (In Russian).
Solntseva
G.N. 1983. Early embryogenesis of the peripheral part of
the auditory analyzer in
a representative of toothed
cetaceans, Stenella attenuata. Ontogenesis, 14 ( 3 ):
312- 318 (In Russian).
Solntseva G.N. 1992. Prenatal development of the peripheral part of
the auditory system in mammals of different ecologies. In: “Marine Mammal
Sensory Systems” (Eds. Thomas, J.A., Kastelein, R.A. and Supin, A.). Plenum
Press, New York, pp. 179 -195.
Solntseva
G.N. 1999 a. Comparison of the development of auditory and vestibular
structures in a representative of toothed whales, the whale (Delphinapterus
leucas, cetacea: odontoceti). Doklady Biological Sciences
364, 78 – 81.
Solntseva G.N. 1999 b. Development of the auditory organ in
terrestrial, semi-aquatic, and aquatic mammals. J. Aquatic Mammals, 25 ( 3 ): 135 -148.
Solntseva G.N. 2007. Morphology of the Auditory and Vestibular Organs in Mammals, with
Emphasis on Marine Species.. Sofia-Moscow-Leiden-Boston: Pensoft & Brill
Academic Publishers. 244 p.
Wilson J.T. 1914. Observations upon young human embryos. J.
Anat. Physiol., 48: 315.