Experience of Application of the Dissolution Test and
Biowaiver Procedure to Evaluation of the Generic Drugs Represented in Russian
Pharmaceutical Market
Smehova I.Ye., assistant professor, Ph.D., Ladutko
Yu.M., assistant professor, Ph.D.
Saint Petersburg State Chemical Pharmaceutical Academy, Russia
Resume: The application of the Dissolution test and
Biowaiver procedure for the evaluation of pharmaceutical and bioequivalence in
vitro for original (brand name) and generic drugs marketed in Russia is represented
in the article. The received data allows making the conclusion about the
possibility of carrying out biowaiver procedure for the establishment of
bioequivalence for nearly 30% of generics in each of studied INN.
Key words:
dissolution, in vitro, biowaiver,
generic drug
The aim of this article is to share the experience of application of the
dissolution test and biowaiver procedure to evaluation of the generic drugs
marketed in Russia and to state the results of work in this field carried out
in Saint Petersburg State Chemical Pharmaceutical Academy (SPCPA) in the
Department of the Technology of Medicinal forms.
Today generics are widely used in different countries. In Russia their
sales volume is about 80%.
In order to
ensure interchangeability in pharmacotherapy, the multisource product must be pharmaceutically,
therapeutically equivalent and bioequivalent to the comparator product.
Pharmaceutical
equivalence implies the same amount of the same active substance(s), in the
same dosage form, for the same route of administration and meeting the same or
comparable standards.
Dissolution
test is one of the tests to prove the pharmaceutical equivalence of the
generics, it also allows prediction of their efficacy in vivo under some conditions.
The test
technique is issued in the form of state standard as a part of the normative
documentation obligatory for performance at acknowledgement of quality of medicinal
products with the same active component.
Generic
medicines should correspond to the same quality requirements as the brand name
medication.
But in
Russia in last two decades because of the lack of the uniform state standards
many manufacturers worked out their own Dissolution tests for the generics they
produced. This was often connected with inaccessibility of the comparator
product test techniques or with inability of generics to meet the requirements
of the original drugs. Lack of
correspondence is determined by the absence of taking the pharmaceutical
factors into consideration. Substances of other quality or possessing the other
physicochemical properties (such as dispersion, solubility, polymorph
modifications) are often used in generics manufacturing. The differences may be
also caused by qualitative and quantitative composition of pharmaceutical excipients,
by technology, by used equipment, et cetera.
Because of the aforesaid verification of generic medicines in all kinds
of equivalence and working out appropriate documentation is of current
importance. It is particularly important for the generics used for treatment of
the socially significant diseases.
Investigation in SPCPA are carried out in the fields of the evaluation
of pharmaceutical equivalence (including the Dissolution testing) and the
establishment of opportunity of the determination of bioequivalence using the
biowaiver procedure.
22 international nonproprietary name (INN) immediate release generic
tablets, coated tablets, capsules of domestic and foreign production were
chosen as the objects for the pharmaceutical equivalence estimation
(amlodipine, ampicilline, atenolol, acyclovir, verapamil, diclofenac sodium,
drotaverine, ibuprofen, indapamide, indometacin, acetylsalicylic acid, co-trimoxazol,
metamizol sodium, metoclopramide, metronidazol, nifedipine, papaverin,
paracetamol, ranitidine, rifampicin, trimetazidine, furosemide). These
medications are referred to different clinical-pharmacological groups. The
biggest part of the objects is included in the WHO list of essential medicines
[1].
It was established that only 25% of methods and requirements of
generic’s Dissolution tests correspond to the same of the original medicines.
Some manufacturers try to obtain the correspondence of
their generics to the requirements of the comparator product’s dissolution test
changing the conditions of carrying it out. For that some surface-active
materials or organic solutions are unreasonably added to the medium, or pH is
changed, or the medium is gained in volume, sometimes the rotation speed of the
apparatus or the dissolution time are increased or the dissolution rate is
reduced, etc. Satisfactory results obtained under such conditions can not
guarantee the equivalence of the generic to the original medicine.
We studied
the dissolution of more than 150 domestic and foreign generics by different methods
including the methods of the original medicines or inscribed in the USP [2]. And for all that we compared the
dissolution profiles calculating the difference and the similarity factors. It
was established that generics meet the requirements of their normative
document. And some of them do not fit with the requirements of the comparator
medicine.
The
conditions of the comparator’s dissolution tests usually allow distinguishing
of generics. That’s why this methods are appropriate to be used both in quality
control of the produced medicines and in assessment of pharmaceutical
equivalence.
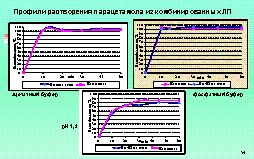
As an
example the results of the analysis of dissolution tests and experimentally
obtained data for verapamil generic tablets 40 mg and 80 mg (“Isoptin” was used
as the comparator product) are represented. On the basis of the similarity
factor the conclusion about pharmaceutical equivalence of 2 out of 6 examined
generics was made.
In our
opinion to prevent the manufacturing of pharmaceutically nonequivalent drugs
and to improve the quality of solid oral dosage forms use of the single
Dissolution test is necessary for the medicines with the same active
ingredient.
Pharmaceutical
equivalence appreciates mainly merchandising characteristics and does not
guarantee therapeutic equivalence. One of the methods to confirm it is the
proof of biological equivalence of the medicines. That is why the other line of
our investigations is the evaluation of bioequivalence.
In most
cases for bioequivalence evaluation pharmacokinetics investigations and in vitro trials are carried out. The
first of them are rather expensive and enduring. That’s why the evaluation of
bioequivalence by in vitro biowaiver
procedure [3] is of current
importance in pharmaceutical practice all over the world including Russia.
For
evaluation the availability of this procedure we carried out grouping into
divisions of biopharmaceutical classification the immediate release solid oral
dosage forms included into the Russian Essential Medicines List and into the
Health Ministry's Additional Medications Provision/Supply program List (picture
1).
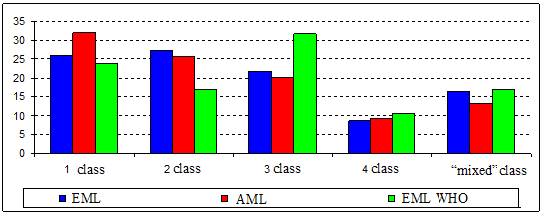
Picture 1. Distribution of immediate release
solid oral dosage forms included into the Essential Medicines List into the
classes of Biopharmaceutical
Classification System (EML - Essential Medicines List; AML -
the Health Ministry's Additional Medications Provision/Supply program List; EML
WHO - WHO Essential Medicines List)
It was
established that there is of about 50% of single-component immediate release
solid oral dosage forms in the Essential Medicines List whereas there are about
80% of them in the Health Ministry's Additional Medications Provision/Supply
program List. The class is known for almost 51% of drugs included into the
Essential Medicines List and 38,2% of drugs included into the Health Ministry's
Additional Medications Provision/Supply program List. We rated to the “mixed”
group the medicines the class for which is not specified yet. For comparison the
data obtained by other authors for the WHO Essential Medicines List are
represented in the picture [4, 5].
Multicomponent
medicines for the active ingredients of which the class has been determined are
prospective for the evaluation of bioequivalence using the biowaiver procedure.
So we
suppose that the bioequivalence for more than 50% of medicines of the 1 and 3
BCS classes included into the aforenamed Lists may be evaluated by biowaiver
procedure. According to the selection scheme the objects for the evaluation of
bioequivalence by biowaiver procedure were chosen. There were the generic
medicines of 9 INN of different BCS classes presented in the Russian
pharmaceutical market (atenolol, acyclovir, indapamide, diclofenac,
drotaverine, metoclopramide, metronidazole, ranitidine, trimetazidine). Some
multicomponent medicines containing ascorbic acid and rutoside, papaverine and
bendazol, drotaverine and paracetamol of domestic and foreign manufacturers
were also chosen. The choice is also caused by the large amount of the generics
of the selected medicines in Russia.
Analysis of
more than 50 medicines of the aforesaid INN appeared that pharmaceutical
substances and excipients in biopharmaceutical and in physicochemical
properties correspond to the essential requirements for bioequivalence in vitro trials.
At the same
time not for all of the investigated generics the dissolution profiles fit with
requirements: while carrying out the in vitro test they dissolved with the
other rate than the comparator product. There was no similarity of the
dissolution profiles in different media. That is why for these generics it is
compulsory to confirm bioequivalence to the comparator product in vivo.
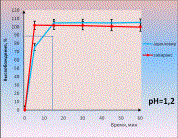
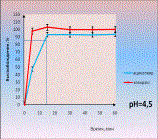
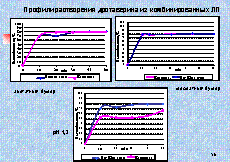
As an
example we give dissolution profiles of acyclovir from tablets (comparator
product is Zovirax) (picture 2). Equivalence of generic medicine to the
comparator was established without mathematical treatment because in all media
there dissolved more than 85% of acyclovir in 15 minutes both from Zovirax and
its generic.
|
|
|
|
Picture 2.
Dissolution profiles of acyclovir in different media
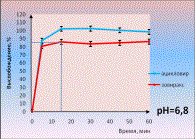
There are
also given the dissolution profiles of paracetamol and drotaverine from
multicomponent medicines with the same composition in three media (picture 3).
Medical products are not equivalent because they have no similarity in
dissolution profiles of drotaverine in one dissolution media.
|
|
|
Picture 3.
Dissolution profiles of paracetamol and drotaverine from multicomponent
medicines
The received
data allows making the conclusion about the possibility of carrying out
biowaiver procedure for the establishment of bioequivalence for nearly 30% of
generics in each of studied INN.
Scientifically
well-founded use of this accessible, easily feasible and reproductible method
permits reduction of financial costs for bioequivalence proof, that is of
current importance for generics used for treatment of prevailing diseases, such
as nonsteroid anti-inflammatory, antiviral, antituberculous, antihypertensive
and others preparations. Especially because many of them are included into the
Health Ministry's Additional Medications Provision/Supply program List and are
covered by public funds.
References
1.
14th WHO Model list of Essential
Medicines, Geneva, World Health Organization, March 2005.
2.
Pharmacopoeia of the United States. The National
Formulary. USP 31/NF 26. - 2008.
3.
Anonymous.”Annex 7”: Multisourse (Generic)
Pharmaceutical Products: Guidelines on Registration Requirements to Establish
Interchangeability. – WHO Technical Report Series, ¹ 937, 2006.
4.
Anonymous.”Annex 8”: Proposal to waive in vivo bioequivalence requirements for
WHO Model list of Essential Medicines immediate-release, solid oral dosage
forms. WHO Technical Report Series, No. 937, 2006.
5.
Takagi T, Ramachandran C, Bermejo M, Yamashita S,
Yu LX., Amidon GL. A provisional biopharmaceutical classification of the top
200 oral drug products in the United States, Great Britain, Spain, and Japan.
Mol pharm. 2006 Nov-Dec; 3(6):631-43.