Áèîëîãè÷åñêèå íàóêè/9. Áèîõèìèÿ è áèîôèçèêà
PhD
Shakhristova E.V., MD, PhD, professor Stepovaya E.A., PhD Nosareva O.L., MD,
PhD, professor Ryazantseva N.V., MD, PhD, professor Novitsky V.V.
Siberian state medical university, Tomsk, Russia
The impact of induced oxidative
stress on cell cycle phase distribution of breast cancer cells
Nowadays
researchers pay special attention to molecular mechanisms of cell system
dysfunction in pathologies which are associated with oxidative stress,
accompanied by redox status change, proliferation dysregulation and apoptosis
[1,2]. Breast tumors are number one
cancer type among women all over the world, and Russia is not an exception. The
objective of the present research is to study the intensity of intracellular
reactive oxygen species production, the degree of protein oxidative
modification as well as cell cycle phase distribution of MCF-7 breast cancer
cells under N-ethylmaleimide-induced oxidative stress.
The research was carried out on the MCF-7 cell line
(human breast adenocarcinoma), obtained from the Russian culture collection at
the Cytology Institute of the Russian Academy of Science (Saint-Petersburg). MCF-7 cells were
cultured in complete growth medium composed of 90% EMEM (“PanEco”, Russia) with
10% (v/v) fetal calf serum (“Invitrogen”, USA), 1% nonessential amino acids
(“PanEco”, Russia), 10 mcg/ml bovine insulin (“PanEco”, Russia), 0.3 mg/ml
L-glutamine (“PanEco”, Russia) and 100 mcg/ml gentamycin (“INS”, USA).
In breast cancer
cells oxidative stress was induced by adding 5mM N-ethylmaleimide (NEM, “Sigma
Aldrich”, USA) to the medium [3] with further culturing for 18 hours at 37°C
and 5% CO2. N-ethylmaleimide irreversibly binds protein and peptide SH groups,
which results in the intracellular oxidant/antioxidant ratio imbalance and
oxidative stress.
The intensity of
free radical oxidation in MCF-7 cells was judged by the concentration of
carbonyl protein derivatives, determined by spectrophotometry (the method is
based on the reaction of oxidized amino acid residues with
2,4-dinitrophenylhydrazine [4]), and the concentration of reactive oxygen
species (ROS), determined by flow cytofluorometry with
2,7-dischlorfluorescein-diacetate (DCFH-DA) fluorescence probe (DCFH-DA, 5mcM,
“Sigma Aldrich”, USA) [5]. Phase
distribution of MCF-7 cells was evaluated by flow cytofluorometry using Cycle
Test Plus kit (“Becton Dickinson”, USA). The results were processed by the
nonparametric Mann-Whitney test.
In the course of the research it was established that
NEM induces intracellular ROS production in breast cancer cells, which was
indicated by the rise in the fluorescence of DCFH-DA-loaded cells (Table).
Table
The concentration
of reactive oxygen species and protein carbonyl derivatives in MCF-7 breast
cancer cells under the effect of N-ethylmaleimide (5mM), Ìå (Q1–Q3)
|
Studied parameters |
MCF-7 cancer cell line |
MCF-7 cancer cell line +
N-ethylmaleimide |
|||
|
Reactive oxygen species,
conventional units |
0,81 (0,80-0,81) |
2,35* (2,25-2,50) |
|||
|
Carbonyl
derivatives of proteins, conventional units /mg protein |
Spontaneous oxidative modification of proteins |
λ=274 íì |
4,52 (3,26-7,34) |
20,21* (13,76-20,61) |
|
|
λ=363 íì |
5,48 (5,01-6,28) |
26,91* (26,22-28,36) |
|||
|
Metal-catalyzed oxidative modification of proteins |
λ=363 íì |
16,34 (15,27-19,38) |
29,88* (29,21-32,16) |
||
|
λ=274 íì |
20,22 (20,09-20,84) |
35,41* (32,72-38,98) |
|||
Note: *
– ð<0,01 statistical significance
calculated with respect to MCF-7 tumor cells. MCF-7 cell culturing in the
presence of NEM resulted in the increase in protein oxidative modification. At
the wavelength of 274 nm aldehyde phenylhydrazones were observed, which are
early markers of protein oxidative modification; at 363 nm ketone
dinitrophenylhydrazones were registered, which are markers of late protein
destruction. Against the backdrop of NEM addition to the cancer cell medium, a
rise (ð<0,01) in the concentration
of carbonyl derivatives was detected at 274 nm and 363 nm under the conditions
of spontaneous and metal-catalyzed protein oxidation, as opposed to the degree
of protein oxidative modification in the intact cells (Table). The increase in
spontaneous and metal-catalyzed protein oxidation is a marker of oxidative
damage to MCF cells, during which proteins act as efficient traps for generated
ROS [6].
It was found out
that the blocking agent of protein and peptide SH groups altered phase
distribution of MCF-7 cells. It was identified that the number of tumor cells in
the S-phase jumped (ð<0,01) under the
effect of NEM due to a fall in their amount in the G0/G1 phase,
as opposed to the intact MCF-7 cells (Fig.).
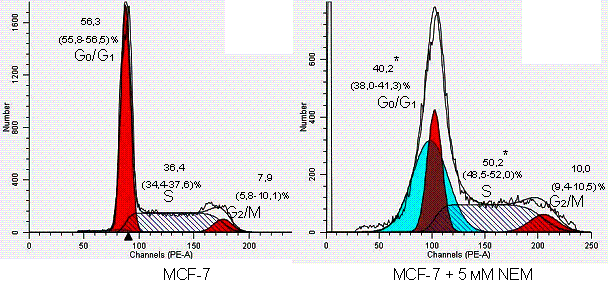
Figure. Cell cycle
phase distribution of MCF-7 tumor cells under the effect of N-ethylmaleimide (*
– ð<0,01 statistical
significance calculated with respect to intact MCF-7 cells).
Therefore, NEM enhanced
tumor cell transition from G0/G1 phase to S-phase,
however it did not significantly change the amount of cells in the G2/Ì phase. Stopping of breast cancer cell cycle in the
S-phase under the conditions of NEM-induced free radical oxidation indicates
violation of DNA replication, which may be associated with alterations in the
functions of redox-sensitive proteins, in particular, transcription factors,
cyclins and cyclin-dependent kinases.
The study was supported by the Russian Foundation for
Humanities as part of the research project No. 15-36-01289.
References:
1. Ryazantseva
N.V., Stepovaya E.A., Nosareva O.L.
et al. Role of heat shock protein 27 in regulation of glutathione system and
apoptosis of Jurkat tumor cells and blood lymphocytes // Bull. Exp. Biol. Med.
2015. 158 (3). 377-379.
2. Murphy M.P.,
Holmgren A.,
Larsson N.G.
et al. Unraveling the biological roles of reactive oxygen species // Cell Metab.
2011. 13. (4). 361–366.
3. Sahaf, B. Lymphocyte surface thiol levels / B.
Sahaf, K. Heydari, L. A. Herzenberg // Proc. Natl. Acad. Sci. ÑØÀ 2003. 100.
(7). 4001-4005.
4. Arutyunyan A.V., Dubinina E.E., Zybina N.N. Metody
ocenki svobodnoradikal'nogo okisleniya i antioksidantnoj zashchity organizma. –
Saint-Petersburg: IKF «Foliant» 2000.
5. Halliwell B., Whiteman M. Measuring reactive species
and oxidative damage in vivo and in cell culture: how should you do it and what
do the results mean? // British J. Pharmacol. 2004. (142). 231–255.
6. Dubininà E.E. Products of metabolism of oxygen in
the functional activity of cells (life and death, creation and destruction).
Physiological and clinical-biochemical aspects. Saint-Petersburg: Medical
press; 2006.