Anodic spark synthesis of ceramic calcium-phosphate
coatings on Ti-6Al-4V alloy from aqueous solutions
Snizhko L.O., Kalinichenko O.O., Misnyankin
D.O.
Anodic spark
deposition on titanium alloys is a fast process for obtaining ceramic coatings
on the surface of valve metals. Finding the interconnection between the
solution composition, time of electrolysis and their influence on the
properties of the layers formed on titanium alloys is a promising area of
research in the field of physical chemistry of medical materials. At the same
time it is necessary to take into account the laws of the layer crystallization
depending on variation of physical and chemical parameters of the experiment
(temperature, pH, time of deposition and electrolyte ingredients
concentration).
The aim of this work
is definition of the
regularities of calcium-phosphate-titanium coatings formation in order to
improve the electrolyte composition and conditions of electrolysis.
Alloy Ti-6Al-4V (90%
Ti, 6% Al, 4% V) widely known as a material for orthopaedics and dental
practice was used as a substrate. Samples were cleaned consequently in 96%
ethanol and acetone in ultrasonic bath during 10 minutes with following washing
in distilled water. Coatings were obtained by one-half-period polarisation
(frequency 50 Hz) at the constant current density 400 A/m2 during 3,
6, 9, 12 and 15 min. After the end of the process, the samples were washed and
dried in the open air. After deposition some samples were annealing at 8000C
for 60 min. Electrolyte solutions were prepared from 2 – 10 g/l Ca3(PO4)2
with 1N Na2EDTA (trilon B) added to the electrolyte to dissolve
precipitate. Formation of soluble complex [CaY]-2 with Y= [(OOC)NCH2CH2N(COO)2]4-
is possible in alkaline media (pH=13), so the electrolyte was adjusted to the
required pH by 2N KOH.
Thickness
measurements and qualitative analysis of coating were performed by scanning electron
microscopy (EDS) at JEOL JSM-6400, which includes an X-ray micro analyser
(NORAN System 7™ X-ray Microanalysis System). Pores distribution was calculated
using ImageJ program intended for treatment of microphotos data with subsequent
processing with Excel built-in function to obtain a normal pores distribution.
Direct measurements of chemical elements concentration were done according to
DIN EN ISO 3497, ASTM B 568 by energy dispersed X-ray fluorescent analysis
(FISCHERSCOPE® X-RAY XDV®-SDD). For X-ray diffraction the
DRON-2 (Ñu -Kα emission) was used.
Fig. 1
represents the main results of the experiment.
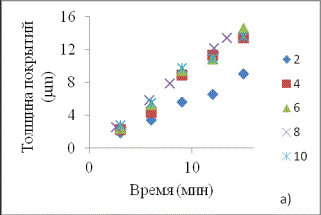
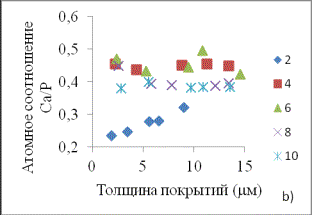
Fig. 1. Dependences of coating thickness from the time of electrolysis (a)
and coatings atomic composition from thickness in the solutions with different
concentration of tricalciumphosphate, g/l: 2, 4, 6, 8, 10.
X-ray diffraction analysis showed the presence of
amorphous phase. The major crystalline compounds were found metallic titanium,
titanium dioxide (anatase), calcium hypophosphite Ca(PO3)2,
tricalcium phosphate Ca3(PO4)2 and calcium
titanophosphate CaTi4(PO4)6. Increasing of
coating thickness caused to the reducing of their porosity. Thus, the maximum
number (~25%) of
small pores with area up to 5 µm2 is observed in thin films with
thickness of 2.5 µm. Otherwise, increasing of coating thickness till 16 µm
reduces the total porosity to 3.1%.
So, the study of
calcium-phosphate coating formation on titanium anode from aqueous solutions by
anodic spark deposition showed that the coatings are composed of substrate elements
(Ti), titanium dioxide in anatase form, as well as calcium phosphate compounds,
such as Ca(PO3)2, Ca3(PO4)2
and CaTi4(PO4)6. Atomic ratio of calcium and
phosphorus does not depend practically on the concentration of the Ca-P
compound in electrolyte. The coating thickness varied between 3 and 15 µm,
atomic Ca/P ratio 0.35 – 0.45 and time of electrolysis 3-15 min.