Detonation characteristics of low-density emulsion explosives sensitized
with polystyrene Foam beads, and their applicability in PERIMETER blasting at
quarries
S.A.
Gorinov
Global Mining Explosive-Russia LLC, Moscow
I.Y. Maslov
Global Mining Explosive-Russia LLC, Moscow
ABSTRACT: It was demonstrated that stable propagation of
explosive process is possible in low-density emulsion explosives obtained
through mixing of emulsion with a significant amount of polystyrene foam (or
other similar) beads. Such process takes place in the form of a detonation-like
wave of emulsion drops combustion in explosive gas streams flowing out of the
high-pressure area of the reaction zone.
Chemical reaction
in such EEs (emulsion explosives) takes places takes place in the form of
surface combustion of emulsion particles interacting with the gas stream.
A method was
developed for determining parameters of decomposition of low-density EEs, and
it was demonstrated that gradual pressure increase takes place in products of
explosive’s decomposition during explosion of such EEs.
Analytical criteria
were obtained for evaluation of propagation stability of this process.
These findings
allow obtaining practically useful results for validation of gentle blasting
technology with the use of low-density EEs.
1. TIMELINESS OF THE STUDY
The use of low-density emulsion explosives sensitized with porous beads
of materials having acoustic stiffness close to the acoustic stiffness of
emulsion (e.g. polystyrene foam beads) may become one of the promising
directions for highly mechanized gentle blasting operations in open cast
mining. Such EEs are not much exposed to shrinkage under hydrostatic pressure
(within the range of charge height of 30-40 meters) [1]. Low densities of such
explosives allow for the efficient use of continuous explosive columns in
gentle blasting [2], which in turn provides a high degree of mechanization of
charging operations.
Previous studies [1,3] have shown that in case the densities of the
examined EEs are higher than 0.75 g/cm3 (for EEs with ammonium
nitrate oxidation phase), initiation of explosives takes place due to heating
of the matrix emulsion matter during its inflow into surface pores of
polystyrene foam beads under the pressure in detonation front.
In case the densities of the EEs sensitized with polystyrene foam beads
are less than 0.75 g/cm3 (for EEs with ammonium nitrate oxidation phase),
deviations were noticed between calculated and experimental values of velocity
of detonation (VOD) [1].
In order to explain the deviations between the calculated value and the
experimental value of VOD at the density of the polystyrene foam sensitized
emulsion explosive less than 0.75 g/cm3 (for EEs with ammonium
nitrate oxidation phase), the following hypothesis was suggested:
a) in case the EE
under study has low density, a connected “polystyrene foam beads – air pores”
system appears within the EE;
b) presence of
end-to-end channels changes the EE’s initiation mechanism. In this case,
initiation will take place according to the mechanism described in study [4] –
under the action of high-enthalpy gas stream filtering from the area of high
pressure. At that, chemical reaction takes place in the form of surface
combustion of explosive particles interacting with the gas stream.
2. STUDY MATERIALS AND RESULTS
In order to validate these provisions, experimental and theoretical
researches were performed.
As a part of field research, experiments were carried out on measuring completeness
and velocity of detonation in the process of blasting of open cylindrical
charges of the examined EE in cardboard tubes (shells). Shells were made of 1mm
thick sheets of laminated electrical cardboard, which were winded in three laps
onto the previously prepared cylindrical templates. VOD Mate (Instantel) and
HandyTrap (MREL) hardware continuously measuring the resistance value of the
conducting sensor’s electrical circuit was used for measuring VOD. Conducting
sensor was glued onto to the cardboard sheet before winding onto the template.
After winding onto the template, cardboard sheets were fixed with a scotch
tape. After template extraction, one of the ends of the resulting tube was
filled with Markoflex polyurethane foam, and after that the shell took its
final form (the “plug” of the solidified PU foam was preventing the Emulpor
from flowing out of the shell; a shell like this can be easily moved across the
field). The shell’s length was not less than 1000 mm, the length of the
chargeable (with EE) part of the shell – not less than 900 mm.
Let’s take an in-depth look at one of the series of experiments.
The emulsion of the following chemical composition was used to create
the EE: NH4NO3 – 75.0% of the total mass, H2O
- 18% of the total mass, emulsifier – 1.0% of the total mass, machine oil –
6.0% of the total mass. The emulsion's density at this chemical composition was
1328 kg/m3 (based on laboratory measurements).
In order to sensitize the above-mentioned emulsion, popcorn beads of 40
kg/m3 bulk density and
8.0mm average diameter were used (these beads have porosity and mechanical
characteristics that are close to such of the polystyrene foam of similar bulk
density). The EE was prepared by mixing of emulsion (EM) with popcorn beads
(PPC) at volume ratio: 7 EM and 4 PPC. Mixture density was 600 kg/m3.
Let’s introduce some designations:
ψ - ratio of the
bulk volume of popcorn beads to the volume of matrix emulsion;
ρ00 - density of the
EE sensitized with popcorn beads;
ρem - matrix emulsion density;
ρppc - bulk density of popcorn beads.
In the case under study, ψ =1.75; ρ00 = 600 kg/m3; ρem = 1328 kg/m3; ρppc = 40 kg/m3.
According to [5], if condition
(1) is true, then a connected “sen›sitizing beads – air pores” system appears
within the EE, and the explosive under study has “foggy” structure consisting
of matrix emulsion drops, which are separated from each other by air pockets
and (or) light, fragile beads.
Packing factor of popcorn
beads was defined through laboratory research and amounted to kpack = 1.6.
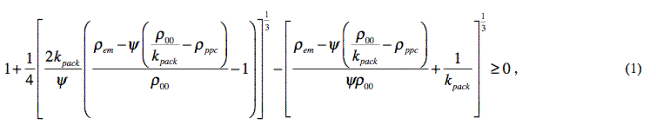
After inserting the given
parameter values in (1), we get 0.089 > 0.
Thus, the EE in the series of
experiments under study had “foggy” structure consisting of matrix emulsion
drops separated from each other by air pockets and (or) light, fragile beads of
the material having acoustic stiffness close to the acoustic stiffness of the
matrix emulsion.
The external appearance of
experimental charge in a cardboard shell of 130 mm diameter is shown in Figure
1.
As the result of experiments,
it was determined that when the composition is initiated by boosters made of
T-1000-L-PO trinitrotoluene blocks, it detonates completely. At that, VOD
amounts to 4123 m/s when blasting 240 mm diameter charge (Figure 2), and to
4065 m/s when charge diameter is 130 mm (Figure 3).

Figure 1. External appearance of experimental
charge in a cardboard shell of 130 mm diameter
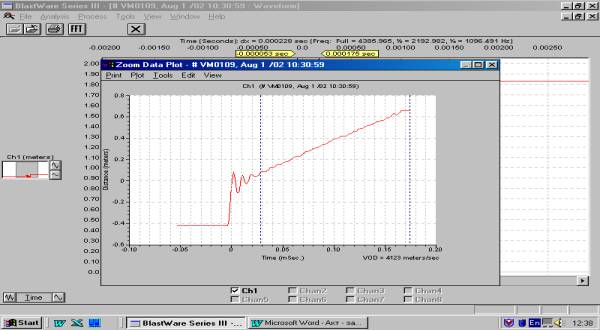
Figure 2. Time variation of the distance covered by the detonation wave
Charge diameter – 240 mm. VOD =
4123 m/s. Booster – T-1000-L-P block
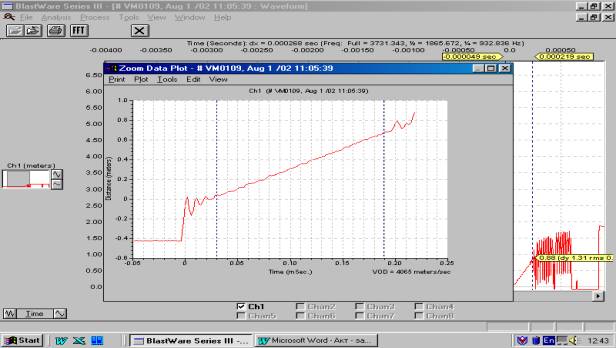
Figure 3. Time variation of the distance
covered by the detonation wave
Charge diameter – 130 mm. VOD =
4065 m/s. Booster –T-1000-L-P block
3. DISCUSSION OF EXPERIMENTAL DATA
Let us assume that detonation processes are described by a model that was
offered in studies [6-9] for description of detonation of industrial ammonium
nitrate explosives. Such model was chosen because it considers the fact that no
instant decomposition of explosive takes place during propagation of detonation
wave within the above-specified explosives. During the initial period,
compression of the explosive takes place under the action of detonation wave as
a result of pore space filling with the explosive, then the explosive is heated
due to internal friction and heat of gases in the pore space, and only then the
explosive ignites. At that, the model considers the fact that the medium acquires
additional velocity during compression along the direction of the detonation
wave propagation, which allows us to explain a number of previously
incomprehensible kinematic effects during propagation of detonation in ammonium
nitrate explosives [6].
Within the scope of the assumed model for description of detonation
process, we have:
according to the energy conservation law, assuming incompressibility of
solid reaction products (within the scope of two-polytropic model for
description of detonation products expansion):
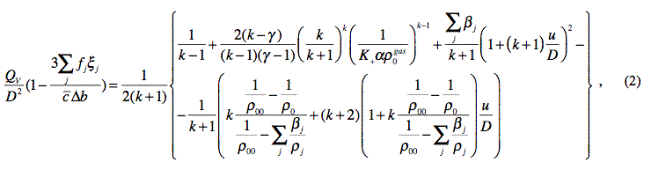
where ![]() is the relative molar heat capacity of
explosion products;
is the relative molar heat capacity of
explosion products;
k is the factor of
polytropic curve of explosion products;
γ is the factor of
adiabatic curve of explosive gases;
![]() is the total part
of solid matter in explosion products;
is the total part
of solid matter in explosion products;
![]() is the total relative volume
of solid explosion products;
is the total relative volume
of solid explosion products;
ρ0gas is the density of gaseous explosion products
at the beginning of EE decomposition;
K+ is a parameter representing the volume of
gaseous explosion products in the conjunction point in case of two-polytropic
description P=P(V) of gaseous explosion products (based on processing of
empirical data, K+»4.4 [6]);
u is the velocity increment of
moving explosion products in the detonation front;
ρ0 = kp ρ00 is the density of the explosive at the beginning of
decomposition (kp is the compression
factor of the explosive at the beginning of its decomposition);
Qv is the specific
heat of reaction;
cv is the specific
heat capacity of explosion products;
α is the covolume of
explosion products (acc. to Vlasov);
Initial density of gaseous explosion products:
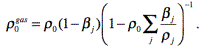 (3)
(3)
![]() value is determined
under the momentum conservation law, and in this case, it approximately equals
to:
value is determined
under the momentum conservation law, and in this case, it approximately equals
to: ![]() (4)
(4)
Explosion gases state equation
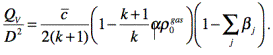 (5)
(5)
Velocity of detonation![]() (in coordinate
system moving along the direction of the process with
(in coordinate
system moving along the direction of the process with![]() speed) equals to:
speed) equals to:
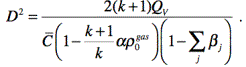 (6)
(6)
The measured VOD (velocity of detonation in the laboratory system of
coordinates) equals to:
![]() (7)
(7)
The system of equations
(2)-(5) can be solved if the value of compression factor kp is known.
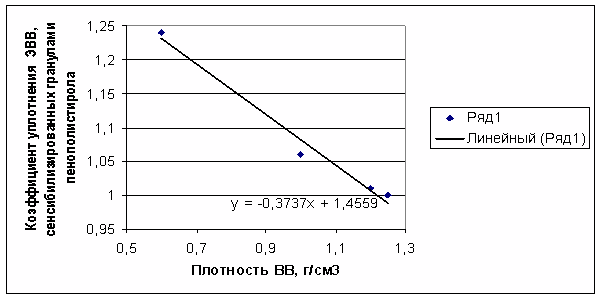
In our case, kp evaluation was
performed through extrapolation of calculated kp values for the
range of Emulpor densities 1.0-1.27 g/cm3 (kp was calculated by
the procedure described in studies [1,3]) to the area of low charge densities.
The extrapolation curve is shown in Figure 4. Based on the acquired results, we
took
kp ≈ 1.45 –
0.37ρ00 for evaluative
calculations, where ρ00 was taken in g/cm3.
At ρii = 0.6 g/cm3, kp = 1.23.
The results of VOD
calculation through the above-given equations for the series of experiments
under study:
![]() = 3195 m/s (D = 2622 m/s; u = 572 m/s).
= 3195 m/s (D = 2622 m/s; u = 572 m/s).
The calculated value is significantly lower than the experimental value.
Thus, the assumption that detonative
decomposition of the EE under study takes place due to the internal friction in
the process of pore space filling and due to the heat of gases in the
compressing pores doesn’t allow us to explain such high VOD values at such a
low EE density.
Hence, another concept is necessary in order
to explain the observed VOD values.
In accordance with (1), there is a connected bead-air system in the
low-density EE under study. Let us assume that initiation of detonation in this
EE will take place in accordance with the explosive initiation mechanism
described in study [4].
In this case, initiation of
the mentioned EE will take place under the action of high-enthalpy gas stream
filtering from the area of high pressure. At that, chemical reaction takes
place in the form of surface combustion of explosive particles interacting with
the gas stream.
Let’s determine the parameters
of the filtering stream of explosive gases from the area of high pressure.
Let’s introduce the following designations:
![]() - stream speed with respect to
a stationary observer;
- stream speed with respect to
a stationary observer;
ρf , Tf - density and temperature of explosive gases
in the head end of the stream of explosive gases flowing out from the area of
high pressure;
ρx , Tx - density and temperature of air in the head
end of the air blast wave generated by the stream of explosive gases.


![]()
![]()
Figure 4. Compression factor of the EE sensitized with polystyrene foam
beads as a function of the EE’s density (extrapolation).
In terms of gas dynamic
relations [10], within the scope of two-polytropic model of explosive gases
expansion, we have the following equations for determination of parameters of
the explosive gas stream filtering from the area of high pressure:
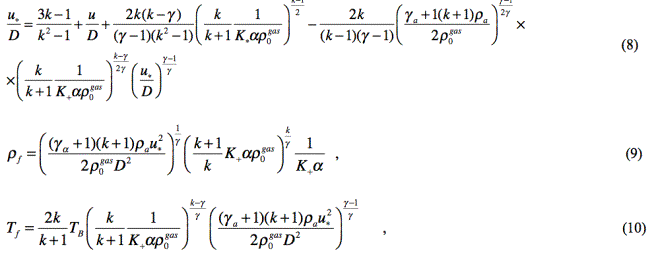
where γa =1.2 is the adiabatic exponent
of air in the blast wave;
TB is the temperature of explosion products.
The stream of explosive gases
flowing out from the area of high pressure generates a blast wave in the air
that is between the beads and inside them (porosity is 95-98%). This blast wave
and the following stream of hot explosive gases flow around the drops of matrix
emulsion “fog”. If these drops inflame during the period less than the duration
of explosion decomposition process in the area of violent chemical reaction
(the area of high pressure), then propagation of the explosive process
throughout the low-density EE will be determined by the propagation speed of
this ignition process.
We shall evaluate the ignition
time of emulsion drops according to study [11].
Induction time τ of ignition of an emulsion drop (when it is
blown over by the air blast wave and the stream of explosive gases) will be
found from the following equations (we assume that due to exceptionally short
duration of ignition process, radiant energy plays the leading role in its
realization):
![]() (11)
(11)
where ![]() is the temperature difference;
is the temperature difference;
![]() - the “reaction
speed – temperature” correlation index; TH - the initial temperature of emulsion; TS - the temperature
of an emulsion drop’s surface, when it is blown over by the air blast wave and
the stream of explosive gases;
- the “reaction
speed – temperature” correlation index; TH - the initial temperature of emulsion; TS - the temperature
of an emulsion drop’s surface, when it is blown over by the air blast wave and
the stream of explosive gases; ![]() - activation energy; z - pre-exponential
factor; c - specific heat
capacity of the matrix emulsion; R - universal gas constant.
- activation energy; z - pre-exponential
factor; c - specific heat
capacity of the matrix emulsion; R - universal gas constant.
We determine the temperature
of an emulsion drop’s surface with regard for deceleration of the blast wave
and the explosive gas stream, and for thermal activity of substances that take
part in heat transfer process [12]:
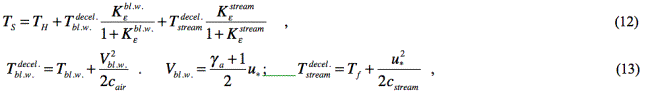
where ![]() are factors of thermal activity of
(correspondingly) air in the blast wave, and the stream of explosive gases with
relation to the emulsion matter:
are factors of thermal activity of
(correspondingly) air in the blast wave, and the stream of explosive gases with
relation to the emulsion matter:
![]()
λ1 , cair , ρx - heat conductivity factor, heat capacity and
density of air (in the blast wave);
λ2 , cstream , ρf - heat conductivity factor, heat capacity
and density of explosive gases in the stream’s head end;
λe , ce , ρe - heat conductivity factor, heat capacity and
density of the emulsion;
Tbl.w. - temperature of
air in the blast wave (it is found from the solutions of [10]).
The condition for
occurrence of detonation transmission by the streams of explosive gases flowing
out from the area of high pressure will take place in case
τ < tchem.r. ,
(14)
where tchem.r. - duration time of
chemical reaction in the area of high pressure.
In the case under
consideration
![]() (15)
(15)
where Rdr is the average
radius of an emulsion drop; vcomb. - the velocity of ablation
combustion.
The velocity of
ablation combustion is determined with reference to the data from [4]:
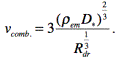 (16)
(16)
The resulting
relations (8)-(10) allow us to evaluate the propagation speed of the detonation-like
process, and equations (11)-(16) allow to evaluate the possibility of process
transmission by the streams of explosive gases flowing out from the area of
high pressure. However, it is necessary to consider the stability of this
phenomenon.
According to V.S.
Trofimov, detonation process will not be disrupted due to pressure drop in the
discharging wave following after the chemical reaction zone if the condition
[13] is true:
![]() (17)
(17)
ς - the part of the
reacted EE matter;
v - specific
volume;
![]() - density of
explosion products in Chapman-Jouguet point;
- density of
explosion products in Chapman-Jouguet point;
θ - the factor of thermal expansion of explosion products
in the reaction zone;
Based on transformation of
thermodynamic relations [14], it may be shown that
![]() (18)
(18)
Let’s substitute (18) into
(17) and take integral. As the result, we get the following condition of
absence of detonation process disruption:
![]() (19)
(19)
In accordance with
[15], the state of detonation products in the area of violent chemical reaction
approaches to liquid properties. Due to the absence of experimental data for θ, this value has been determined according to
the formula [16,17]
![]() (20)
(20)
where μ is Poisson ratio (in this case, μ = 0.5); Csound - sound velocity in
explosion products in the reaction zone.
On the basis of (6), (19),
(20), we get the following criterion of absence of detonation disruption:
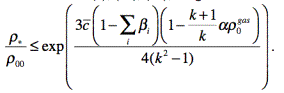 (21)
(21)
In rough figures, we have the
following expressions for some parameters that are included in (21):
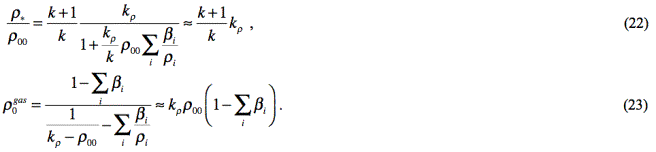
On the basis of (21)-(23), we
get the following criterion expression for evaluation of absence of detonation
disruption:
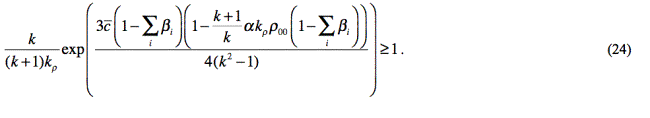
4.
RESULTS OF CALCULATIONS USING THE MODEL OF EXPLOSIVE PROCESS STREAM TRANSMISSION
While performing the calculations,
we assumed that emulsion ignition starts from exothermic decomposition of
ammonium nitrate. Then, the values of activation energy ![]() and pre-exponential factor z in formulas (11) may be defined equal to [12]:
and pre-exponential factor z in formulas (11) may be defined equal to [12]: ![]() = 169.5 kJ/mol, z = 6.8 * 1013 s-1. The value of heat
capacity of the matrix emulsion was determined in accordance with [18].
= 169.5 kJ/mol, z = 6.8 * 1013 s-1. The value of heat
capacity of the matrix emulsion was determined in accordance with [18].
Polytropic curve factor –
1.907, adiabatic curve factor – 1.289;
Heat of explosion – 596.5
kcal/kg; specific volume of gases – 1093 l/kg;
Density of explosion gases in
the head end of the stream – 0.0457 g/cm3;
Temperature of explosive gases
in the head end of the stream – 628 ºK;
Pressure in explosive gases in
the head end of the stream – 20.9 MPa;
Pressure in the area of
violent chemical reaction – 1.69 GPa
Velocity of the gas
stream flowing out from the area of high pressure – 3837 m/s;
Velocity of the air
blast wave propagating in front of the stream – 4221 m/s.
Duration time of
chemical reaction – 56.2 μs; Induction time of emulsion ignition – 51.7
μs;
Criterion of
absence of reaction disruption – 0.982 (~1).
Hence, the idea of the low-density EE initiation by the streams of
explosive gases flowing out from the area of high pressure allow us to obtain
numerical values of VOD that are close to the experimental values.
5. INDUSTRIAL EXPERIMENTAL
TESTING
Industrial-experimental testing of possibility to perform perimeter
blasting using a low-density emulsion explosive sensitized with polystyrene
foam beads was carried out by OAO “Uralasbest” (OJSC) through
industrial-experimental blasting of 141/15-center block [1].
The EE was prepared by
mixing-charging machine TSZM-11 (ÒÑÇÌ-11) in the process of hole charging.
During the experiment, 98 dry
holes of 115 mm diameter were blasted.
Length of holes – 16.5 m;
length of charge – 12.5 m. Charging density – 0.485 g/cm3.
Instantaneous initiation.
Presplitting quality when
blasting with low-density EEs is comparable with blasting quality in the
process of presplitting with garland charges.
Despite its low density (less than 0.5 g/cm3), the EE has preserved the
ability for decomposition.
6.
CONCLUSIONS
In low-density EEs
sensitized with polystyrene foam (or other similar) beads, it is possible to
provide stable propagation of detonation-like wave of emulsion drops combustion
in explosive gas streams flowing out of the high-pressure area of the reaction
zone.
At that, gradual
pressure increase is observed in explosive decomposition products, which is
advantageous for performance of gentle blasting.
A method was
developed for determining parameters of decomposition of low-density EEs.
Analytical criteria
were obtained for evaluation of propagation stability of this process.
The research
results allow obtaining useful information for creation of new types of
low-density EEs, and validation of gentle blasting technology with the use of
such EEs.
ACKNOWLEDGEMENT
Authors express
their gratitude for useful discussions of the issues under study to Doctor of
Engineering Sciences, Associate Member of the Hungarian Academy of Sciences,
V.V. Andreev.
REFERENECES
1. I.Y. Maslov. Increasing the efficiency of
blasting preparation of enclosing rocks at Kuzbass open casts with the use of
emulsion explosives sensitized with polystyrene foam beads / Ph.D. thesis in
Engineering Science, major 25.00.20, M., 2013. – p.132.
2. M.S. Shalaev, G.P. Paramonov. On the
question of increasing the efficiency of screening slot by selecting a rational
design of perimeter charge. Blasting work No.103/60. M.: Inter-Departmental
Commission on Blasting Works at Mining Science Academy, 2010. pp.190-199.
3. S.A. Gorinov, I.Y. Maslov. Evaluation of detonation parameters of
emulsion explosives sensitized with plastic polymicrospheres: Individual
articles of Mining Research and Information Bulletin (scientific and technical
journal). – 2011 – No.7. – pp.53-63. - M.: Publisher “Gornaya Kniga” – 2011
4. V.V. Andreev, A.P. Ershov, L.A. Lukyanchikov. Two-phase low-velocity
detonation of porous explosive / Physics of combustion and explosion, 1979,
vol.15, No.1, pp.89-93.
5. I.Y. Maslov. Evaluation of characteristic density of emulsion
explosives sensitized with polystyrene foam beads / Mining Research and
Information Bulletin (scientific and technical journal). – 2014. – No.6.-
pp.3-10. .- M.: Publisher “Gornaya Kniga” – 2014ã.
6. S.A. Gorinov. Approximating method of detonation parameters
calculation for low-density ammonium nitrate explosives // Mining Research and
Information Bulletin (scientific and technical journal), 2010, No.10,
pp.244-256.
7. Theoretical evaluation of influence of oxidizing agent’s chemical
nature on detonation characteristics of emulsion explosives / S.A. Gorinov,
V.P. Kuprin, I.L. Kovalenko, E.P. Sobina – In the book: Developing resource
saving technologies in blasting works. - Ekaterinburg, pub. Ural Branch of the
Russian Academy of Sciences, 2009, pp.191-201.
8. S.A. Gorinov. Theoretical evaluation of detonation parameters of
granemites. // Mining Research and Information Bulletin (scientific and
technical journal), 2010, No.8, pp.121-130.
9. B.N. Kutuzov, S.A. Gorinov. Physical and technical basics of creation
of emulsion-type and granular explosives, and the means for their initiation.
// Mining Research and Information Bulletin (scientific and technical journal),
2011, No.7. Preprint. pp.34-52.
10. F.A. Baum, K.P. Stanyukovich, B.I. Shekhter. Physics of explosion.-
M.: pub. Fizmatgiz, 1959. p.800.
11. V.N. Vilyunov. Theory of ignition of
condensed substances.- Novosibirsk, Science, 1984.- p.189.
12. Theory of combustion and explosion. P.P. Kukin, V.V. Yushin, S.G.
Yemelyanov, et. al. – M., pub. Urait, 2014.- p.435.
13. A.N. Dremin. Pulsating detonation front / Physics of combustion and
explosion, 1983, No.4, vol.19, pp.159-169.
14. V.G. Levich. Course of theoretical physics. Vol.1.- M.: Science,
Chief Editorial Board of Physics and Mathematics Literature, 1969.- p.912.
15. L.D. Landau, K.P. Stanyukovich. On studying detonation of condensed
explosives. / Reports of the USSR Academy of Sciences, 1945, ò.46, ¹9,
ñ.399-402.
16. V.N. Belopomestnykh. Acoustic Grüneisen
parameter of solid bodies // Letters to Journal of Technical
Physics.- 2004.- Vol.30, issue no.3, pp.14-19.
17. E.P. Tesleeva. Research of polymorphic transformations in
ion-molecular dielectric materials through methods of physical acoustic and
thermal physics // Author’s abstract of the Ph.D. thesis in Physics and
Mathematics, Barnaul, 2006.- p.22.
18. S.A. Gorinov, I.Y. Maslov. Thermal-physical properties of
non-sensitized emulsion matrix – a component of emulsion explosives // Mining
Research and Information Bulletin (scientific and technical journal).
Individual articles.- 2011.- No.12, pp.17-20. - Ì.: pub. “Gornaya Kniga”.