THE
PHYSICAL-CHEMICAL CHARACTERISATION OF COMPOUNDS BASED ON SILICON WITH ALUMINIUM
CHLORIDE USING SULPHUR, DERIVED USING THE MOLECULAR STRATIFICATION METHOD
Zh.S.Yessenamanova,
T.K.Ahmedzhanov, M.S. Yessenamanova,
C.C.Nurkeev ,
B.M.Nuranbaeva
K.
I. Satpayev Kazakh National Technical University, Almaty
Kh.Dosmukhamedov
Atyrau State University, Atyrau
Thesis
This work considers the possibility of deriving
nanostructure and sulphurous compounds using an electrophilic reagent,
aluminium chloride, using sulphur by the molecular stratification method.
Molecular stratification technology makes it
possible to derive active centres of nanostructures of chlorides chemically
bound to transitional d-elements on the surface of amorphous silicon.
1.
Introduction. One of the new chemical
nano-technological processes which is well known both in Russia and abroad,
based on the carrying out of chemical reactions on the surface of various solid
phase matrices is the molecular stratification method (MS) [1-3] which has been
named in the work of foreign authors by the not totally adequate name of atomic
layer epitaxy (ALE), and atomic layer deposition (ALD).
Based on the
chemical assembly of nano-structures on the surface of solid bodies using the
molecular stratification method (MS) is the idea, developed by the school of
head correspondent of the Russian Academy of Sciences, V. B. Aleskovskii [1] in
the field of chemical supramolecular bonding. In the fifties and sixties, based
on the ‘spanning hypothesis’ of V. B. Aleskovskii and his students [1], a
precision chemical method of synthesis of solids was developed which was given
the general name of ‘chemical assembly method’.
The
transformation of supra-molecular substances by means of the interaction of
functional groups laid the foundation for the forming of the precision method
of synthesis of solid materials – the molecular stratification method. The
principles of the MS method were the first to be proposed by him together with
S. I. Kolstov at the beginning of the sixties [2]. In ensuing publications
(between the sixties and the eighties) a more in-depth consideration of the
chemical basis for the new method of synthesis of solids was shown by authors
and their students such as A. N. Volkovii, R. R. Rachkovskii, V. M. Smirnov, A.
A. Maligin and others [3].
The basic
idea of the MS method which is used for the precision synthesis of solid bodies
with a regular construction is the sequential building up of single layer
structures of the said chemical composition on the surface of solid phase
matrices.
The outline
of the process of the chemical assembly of nano-structures on the surface of
solid bodies using the MS method, as well as the analysis of existing
experimental data, confirms the fact that the MS method can be used to synthesise
nanostructures with various chemical compositions (mono layer, including
multi-component) on the surface of
solid phase matrices, as well as to carry out atomic chemical assembly
on the surface of nano-, micro- and macro- structures by means of multiple
alternation of chemical reactions of the said programme. It should be noted
that the MS method guarantees the formation of layers on the surface of solid
to an accuracy of within one single molecular layer.
At the
present time, a significant number of chemical transformations using centres on
the surface of silicon have been carried out. As a result of chemical
modification it has been possible to form new practically important, high
specification adsorbents, selective catalysts, active polymer fillers and
efficient viscosifiers for dispersants.
Very
frequently one type of chemical reaction using particular centres of surfaces
is carried out in totally different conditions. This can be related to the
particularities of the electrical and geometric construction of the modifying
agents present in the reaction volume of electron and proton molecules, and the
gradual reduction and hydroxylation of the surface of the original silicon,
diffusion and other factors.
It has been
shown that all chlorides, with the exception of CCl4, when heated to
a temperature of about 180oC, begin a condensation reaction with
polysilic acid, in the process losing at least one chlorine atom [3].
Molecular
stratification is known as one of the synthesis methods for solids by means of
assembly on a matrix of structural units. By this means it is possible to
derive multi-zone solids, regulating the order of the layers which are placed,
as well as the thickness of the layer to one monolayer that is within the
achievable accuracy.
Among the
reactions which have been already studied, with the participation of functional
groups on the surface of silicons, it has been possible to separate out
neutrophylic and electrophylic displacement (SN, SE),
neutrophylic and electrophylic bonding (AdN, AdE, AdN,E),
as well as elimination processes (E) [3].
Possible
types of heterolytic transformation with the participation of centres on the
surface of the original silicon can be shown by the diagram in figure 1 [3].
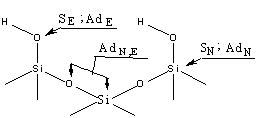
Figure 1 – A diagram of
the heterolytic transformation using centres on the surface of the silicon.
First of all,
it is possible to separate a large group of reactions, in which the attack is
made by electrophylic agents on oxygen atoms with a silanol group surface
structure. This is a reaction of electrophylic displacement of a proton with
the interaction of various chlorine and alkoxysilane, organosilazanes and other
elementary organic compounds, for example isocyanates [4].
For the
reaction of electrophylic displacement of a proton on silanol group surfaces it
is postulated that four-centred transitional compounds are formed. In this
case, the degree of nucleophylic action on the part of the one being
substituted out (X) is very important. The depth of such interaction must be
determined by the proton acceptor composition X [4].
The synthesis of multi-component mono-layer
oxides can be carried out as follows [6]:
-
The sequential treatment of matrices with chloride gases;
-
The substitution on the surface of elements of the
oxochloride group in reaction with chlorides of another chemical nature;
-
The treatment of the substrate mixture of chlorides with
other elements;
In the
literature, a description is given of a significant number of processes for
inorganic sulphurous compounds from solutions. In order to regulate the
composition of the materials which are derived from the sulphurous liquids,
modifying agents are used, in the form of various organic and inorganic
compounds. The use of modifying agents is one of the best known methods of
managing the production of sulphurous construction materials.
In the
production of sulphur containing materials in the form of fillers for a wide
range of uses, various silicon modifiers are used: sand (a-quartz) and rich
specific surfaces of amorphous silicon (silicon gel, aerosil). In the
literature of the USSR, the technology for deriving compounds using vibration
with preloading, analogous with factory technologies for cement concrete [5]
has been developed. The filler is mixed with ground sulphur, and then formation
is carried out, using vibration under conditions of pre-loading of 50-500g/cm2,
after which the product is heated for 1-3 hours. The solidity achieved under
pressure using a granite filler and ground quartz sand is 50-80MPa [5].
As follows
from above, the range of contemporary sulphur compounds based on low molecular
crystallised and polymerous sulphur is significant, and of various quality and
purpose. In all publications shown the technological processes and the composition
of the materials derived are shown. However, there is a practical lack of
information on the mechanism of the interaction of components in these
processes and there is no information on the chemistry of the processes. The
ecological aspects of such have also not been considered. Meanwhile, it is
well-known that the heating of sulphurous compounds to temperatures of melting
always lead to the formation of sulphur dioxides. The sublimation of sulphur
takes place at a temperature of 7oC and its oxidisation begins at a
temperature of around 100 oC.
An analysis
of literature sources shows that the majority of research has been dedicated to
the study of the influence of modifying additives on the composition of the
sulphurous compounds, including sulphur, fillers and aggregates and very little
work has been done on the study of the composition sulphur in the presence of
various active agents. This makes it difficult to make an informed choice of
the most effective modifiers in each specific instance. Thereby, research into
the influence of various modifiers on the composition of derivatives and the
stud of ecological aspects of processes involving non-organic sulphides and
materials is a pressing task.
2.
Results of molecular chemical calculations.
In order to
explain the possibility of deriving nano-structures on the surface of silicon
gel with modification using aluminium chloride, molecular chemical research was
first carried out.
An important
pre-condition for successful molecular chemical research is the correct choice
of a calculation method, first of all the means of calculation of electronic
correlation and the foundation used. The first test which various molecular
chemical methods undergo is the accuracy of the communication of the character
of the energy change in the event of the disruption of links between various
molecules. The second is the determination of the accuracy of the transmission
of geometric structures. The results of the tests and their comparison with
experimental evaluations are shown in Table 1.
Table
1. Dissociation energy (D) and length of connections (r) of various compounds
|
Compound |
Data
from literature |
Calculated
data in RMZ |
Calculated
data in B3LYP/6-31G(d) |
Calculated
data in Priroda |
||||
|
g, PM |
D, kJ/mole |
g, PM |
D, kJ/mole |
g, PM |
D, kJ/mole |
g, PM |
D, kJ/mole |
|
|
S2 |
188.9 |
412,
14+-2.54 05.85+-21 |
185.3 |
439.32 |
192.7 |
400.64 |
193.2 |
467.48 |
|
SH |
134.1 |
340.6+-12 |
129.7 |
331.79 |
135.5 |
341.5 |
136.1 |
363.63 |
|
SO |
148.09+-0.00001 |
516.20+-0.13516.72 |
145.8 |
469.03 |
151.2 |
487.56 |
152.4 |
566.93 |
|
SiS |
192.93 |
619+-12.6 |
179.1 |
581.99 |
195.17 |
572.29 |
196.3 |
614.55 |
|
Si2 |
225.2 |
309.6+-21 288.7+-21 |
182.2 |
307.94 |
217.14 |
283.47 |
218.0 |
324.47 |
|
SiH |
152.1 |
309.6+-25 |
195.5 |
270.7 |
153.9 |
197.82 |
155.1 |
297.06 |
|
SiO |
150.9 |
803.24+-21.34 |
146.6 |
808.77 |
152.4 |
751.45 |
154.0 |
803.24 |
|
AlCl |
212.98 |
482.79+-7.12 |
204.9 |
374.89 |
211.04 |
441.22 |
212.19 |
496.13 |
*Molecular
permanent inorganic compounds: Reference book by K. S. Krasnov, N. V.
Philipenko, V. A. Bokova, et al., one
of the official journals of chemistry of K.S. Krasnov, Chemistryh 1979, page
448.
The thermodynamic
character of the compound which we have determined shows that the calculation
which gives the nearest value to the experimental value is the calculation of
the Priroda programme (3z.bas) and B3LYP 6-31G(d).
According to
the results of bonding, the formation of one or two sulphur atoms is much more
long-lasting. Further, with an increase in the sulphur atoms in the chain, the
energy of bonds (Si—S,
O—S) is reduced
and stabilises. More stable bonds are formed by the joining of sulphur to
silicon atoms (substitution of hydroxyl group).
Priroda makes it possible to reliably
evaluate the mechanism for the bonding in the sulphur system ie silica gel.
This depends on the quality of the method and basis of the programme, taking
into account the d-orbit of atoms. Reactions involving functional groups on the
surface of silica gel, can be divided into those with nucleophyilic
displacement and bonding. This reaction is with the attack of a nucleophylic
agent (sulphur radicals) and nucleophylic displacement of OH- groups or nucleophylic bonding (introduction of
oxygen.
Reactions of bonding
of sulphur to silicon atoms with the displacement of OH groups occur
endothermically (104.14 kJ/mol). In the transitional stage Si–O bonds are
lengthen to 236.5 pm, and O–S and Si–S bonds shrink to 227.7 pm and 229.5 pm, accordingly. The activation
energy for nucleophylic displacement is 120.16 kJ/mol. Reactions for the
introduction of diatomic sulphur (singlets) to oxygen atoms take place
endothermically (9.21 kJ/mol). In the transitional stage the O–H bonds lengthen
to 123.8 pm, and the O–S and S–H shrink to 188.7 and 176.0 pm accordingly. The activation energy is
equivalent to 139.56 kJ/mol. The introduction
of sulphur triplets takes place endothermically (143.13 kJ/mol). The activation
energy is equivalent toà
169.95 kJ/mol. The process of bonding of diatomic sulphur (triplets) to silicon
atoms with the displacement of the OH group takes place endothermically (266.96
kJ/mol). In the transitional stage the Si–O bonds lengthen to 224.9 pm, and the
Si–S bonds shorten to 224.0 pm. The activation energy for nucleophylic displacement is 264.39 kJ/mol.
Thermodynamic
benefits, from the point of view of activation energy and more stable bonding
are generated in the reaction of the introduction of oxygen radicals of sulphur
in the singlet form (Åàêò=134.82 kJ/mol), as well as
the introduction of sulphur radicals in the singlet form to the silicon atom (Åàêò=146.35 kJ/mol). The products of these reactions form stable valent bonds of sulphur with
oxygen atoms (282.8 kJ/mol) and
atoms of silicon (309.9 kJ/mol), determining
the formation of other sulphurous compounds.
The modification of
silica gel by aluminium chloride facilitates an increase in the active centres
on the surface of the silica geland the forming of sulphurous rings.
3. Experimental results.
Laboratory tests to
derive sulphurous compound specimens based on silica gel, sulphur and aluminium
chloride were carried out in order to confirm the theoretical quantum-chemical
estimates. The strengthening of the behaviour of the derived compounds, has
been verified by a hydraulic laboratory using a PGL-5 press, as shown in figure
2.
The preparation of
specimens of sulphurous compounds using aluminium chloride was carried out in
two stages. Firstly, the silica gel was modified using aluminium chloride mixed with reagents with
heating to a temperature of 200 – 500îÑ, then the modified silica gel was introduced to
liquid sulphur. As can be seen from the figure, the dependency of solidity of
the sample on the amount of modifying reagent AlCl3 is very high. The
specimen, after preliminary ignition at 5000Ñ and with an aluminium chloride content of 5% mass has
the optimal value of solidity under pressure. The solidity of such a sample is
equal to 70 ÌPà.
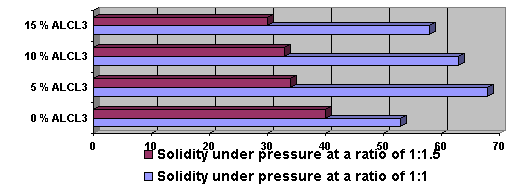
Figure 2. The dependence of the solidity on the pressure of the formation of
sulphurous sompounds from aluminium chloride containing compounds with a correlation of 1:1 or
1:1,5 at
a temperature of initial thermal treatment of 500 îÑ.
Table 1 shows the
results of the physical-mechanical testing of compounds, derived under optimal
conditions, containing aluminium chloride of 5% mass.
Table 2 The physical-mechanical and operational
indices of sulphurous materials (AlCl3 – 5% mass)
|
Relationship
of binding to the filling mass |
Temperature
of the reagent in îÑ |
Density
of the sample, g/ñm3 |
Frost
resistance of the cycles |
Water
absorption % mass |
Shock
resistance, ÌPa |
Resistance
coefficient |
|||||
|
5%
HCl |
5% H2SO4 |
5% CaCl2 |
5% NaCl |
5%
MgSO4 |
|||||||
|
1:1 |
400 |
1.796 |
228 |
2.94 |
34 |
0.989 |
0.949 |
0.959 |
0.977 |
0.963 |
|
|
1:1.5 |
400 |
1.684 |
188 |
4.06 |
21 |
0.928 |
0.920 |
0.928 |
0.933 |
0.929 |
|
|
1:1 |
500 |
1.804 |
239 |
2.46 |
48 |
0.966 |
0.957 |
0.964 |
0.979 |
0.965 |
|
|
1:1.5 |
500 |
1.811 |
202 |
3.74 |
23 |
0.924 |
0.924 |
0.931 |
0.936 |
0.933 |
|
As
can be seen from the table and diagram, compounds produced by the suggested
formulation with an optimal ration of components, have a high resistance
coefficient to the solutions HCl, H2SO4, CaCl2 , NaCl, MgSO4, a high shock resistance (48 ÌPà), frost
resistance (239 cycles) and density (1.804 g/cm3).
Physical-chemical
research was carried out in order to explain the reasons for an improvement in
the physical-mechanical behaviour of the composition of the optimal structure
as a result of possible chemical interaction with the formation of silicon
sulphides.
Results
of IR spectroscopy are shown in Figure
3.
Figure 3. IR-spectrum
of aluminium chloride (1), silica gel
(2), silica gel specimens modified by
5% AlCl3, under various
thermo-treatment temperatures: 3 –
2000Ñ; 4 –
5000Ñ; and sulphur composition based on silica gel
from 5% AlCl3 (5000Ñ) (5).
In the case of aluminium
chloride modified by silica gel, triplets can be seem with an area of 2850-2950
cm-1, which indicates the appearance of new chemical bonds in the system
and the formation of active centres with temperatures raised to 400-500oÑ.
4.
Discussion and conclusions
The results of
physical-chemical research suggest that the high physical-chemical behaviour of
the compounds formed, depend on the chemical interaction of sulphur with
aluminium, secured on the surface of the silica gel, as well as with oxygen and
silicon itself in the form of silica gel by the donor-aceptor mechanism.
5.
Bibliography:
1. V. B.
Aleskovskii, The Chemistry of sub-molecular compounds: Text book, St. P:
Publisher. St. Petersburg University, 1996. p.256
2. S. I.
Koltsov, Chemical Construction of Solids, Lensovet Press, 1990, p.48.
3. À.À. Malygin, Chemical assemblage of materials with the set
properties: The text of lections. L.: LTI, 1986. p. 51
- À.À.
Malygin, U.K. Ezhovsky, The equipment of process
of chemical assemblage of materials: The manual. L.: LTI, 1987. p. 96
- Ph.D. Minerbaeva, M.Sh. Ospanova, Building composition: the invention Description to the
copyright certificate of the USSR 1265175: the opening Bulletin.
Inventions. - 1986.- ¹ 14.