Ph.D., Ziyadullaev O.E.,
prof. Turabdjanov S.M.
masters Juraev R.S.,
Lecturer Abdurakhmanovà S.S.
Tashkent Chemical
Technological Institute, Uzbekistan
SYNTHESIS OF AROMATIC ACETYLENE ALCOHOLS OF
THE METHODS GRINYARA-IOTSICHA
Reacting with
phenylacetylen (FA) croton aldehyde and near ketones in the presence of
organomagnesium compounds synthesized aromatic acetylenic alcohols (AAA).
Organometallic
compound first synthesized by E. Franklin. M. Barbe is shifted synthesized
organometallic compounds [1, 2]. French scientist B. Grinyar reaction of alkyl
halides with anhydrous magnesium metal powder mixed synthesized organometallic
compounds (reagent Grinyara) and developed the conditions of their application
to various organic syntheses. New stable compounds by reaction with alkynes
reagent Grinyara synthesized J. Iotsich [3, 4].
The high reactivity
of C≡C- and C-H bonds in alkynyl
makes them participants in the diverse reactions that form the basis of section
synthetic organic and industrial chemistry [5, 8].
AAA thanks to
important applications are used on a large scale in various industries,
including the oil and gas industry as bio inhibitory products biological
corrosion of metal equipment, as ion exchangers for the purification of
hazardous waste and production of oil and gas [6], as an additive stability at
low for aviation fuel temperatures [7], in paint factories in the light
industry, agriculture, medicine and electrical engineering for various
purposes.
In reaction Grinyara- Iotsicha using croton aldehyde and
ketones (acetone, methylethylketone, methylisopropylketone, pinokaline and
acetophenone) FA by their interaction with the organomagnesium compound
synthesized in the following respective compounds AAA- 1-phenyl-3-methylbutyn-1-ol-3 (I) 1-phenyl-3-methylpentin-1-ol-3 (II) 1-phenyl-3,4-dimethylpentyn-1-ol-3 (III), 1-phenyl-3,4,4-trimethylpentyn-1-ol-3 (IV) 1,3-diphenylbutyn-1-ol-3
(V) and 1-phenylgekcyn-4-in-1-ol-3 (VI).
The scheme of the reaction has been offered as
following
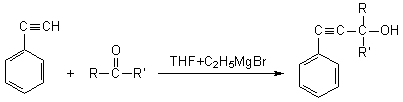
here: R= ‒CH3, R'= ‒CH3;
R= ‒CH3, R'= ‒C2H5; R= ‒CH3,
R'= izo ‒C3H7, R= ‒CH3,
R'=
‒C(ÑH3)3; R= ‒CH3, R'= ‒Ñ6H3,
R= ‒H, R'= ‒ÑH=CH‒CH3.
Synthesis process
AAA method Grinyara- Iotsicha conducted at a temperature range -5-10 °C in the
presence of solvents diethyl ether (DEE) and tetrahydrofuran (THF). The
starting materials were taken in equimolar amounts. The results are shown in
the table.
Table
Influence of the nature and
duration of the reaction solvents on
the yield of AAS (temperature 0 ° -5, starting
materials in an
equimolar ratio)
|
ÀÀA |
Products, % |
|||
|
solvent DEE |
solvent THF |
|||
|
ÀÀA |
intermediate and auxiliary connections |
ÀÀA |
intermediate and auxiliary connections |
|
|
Duration of reaction, 2 hour |
||||
|
I |
75,0 |
18,3 |
87,4 |
6,5 |
|
II |
68,4 |
23,4 |
83,5 |
10,2 |
|
III |
56,4 |
29,5 |
74,5 |
15,3 |
|
IV |
53,0 |
34,7 |
69,3 |
18,4 |
|
V |
50,0 |
39,6 |
66,0 |
21,0 |
|
VI |
64,3 |
26,4 |
79,0 |
14,1 |
|
Duration of reaction, 4 hour |
||||
|
I |
78,6 |
13,2 |
89,6 |
4,5 |
|
II |
71,8 |
17,4 |
85,0 |
7,6 |
|
III |
60,6 |
21,6 |
75,2 |
13,0 |
|
IV |
55,9 |
25,0 |
71,0 |
15,5 |
|
V |
54,7 |
28,0 |
68,7 |
18,2 |
|
VI |
68,0 |
20,0 |
81,2 |
12,2 |
|
Duration of reaction, 6 hour |
||||
|
I |
67,3 |
24,8 |
82,2 |
11,4 |
|
II |
62,4 |
27,0 |
77,6 |
17,0 |
|
III |
52,4 |
37,0 |
69,5 |
18,4 |
|
IV |
45,2 |
41,6 |
63,2 |
21,0 |
|
V |
42,7 |
44,0 |
57,3 |
23,7 |
|
VI |
58,6 |
35,6 |
70,6 |
17,4 |
The table shows that
when using THF as solvent, increasing the reaction time from 2 to 4 hours, an
increase yield. However, with increasing duration of synthesis up to 6 hours, a
sharp fall of efficiency AAA exit.
The table shows that
when the reaction is carried out in solvents DEE and THF in AAA is formed with
a high yield. For example, with a reaction time 4 hours temperature -5- 0 oC in a solvent DEE, yield AAA: I- 78,8; II- 71,8; III-
60,6; IV- 55,9; V- 54,7 and VI- 68,0%, and in case of replacement solvent THF
respectively 89,6; 85,0; 75,2; 71,0; 68,7 and 82,2%. Comparing the reaction
results in different solvents shows that the average selectivity output y THF
solvent by 13,0% more than the DEE. This reaction is due to the greater
polarity of THF molecule compared to DEE.
The reaction carried
out in THF, AAA negligible amount of
by-products and intermediates. Solubility Iotsicha reagent in THF solution is
very high; it creates a comfortable environment for the last phase of collision
with aldehydes and ketones. Halogen AAA salt formed during the reaction is
hydrolyzed, THF serves as a catalyst and, disaggregated electron pairs in its
molecule solvation salts promote conversion AAA. THF relatively low solubility
in water than DEE forms with water an unstable tetramethylglycol in weakly
acidic medium and forms tetrametilenhlorgidrin acid as a byproduct. This
reduces the amount of alkoxides formed by reacting a basic salt AAA and
provides the basis for the synthesis of alcohols in high yield.
To explore possible
areas of application of the synthesized compounds was studied their
microbiological activity against the biological corrosion of pipelines of the
oil industry in the laboratory together with the staff of the Institute of
Microbiology, Academy of Sciences of Uzbekistan
[9, 10].
AAA obtained possess
microbiological activity, among the compounds studied V, I and II have active
antibacterial properties against bacteria Pseudomonas, Arthrobacter
chroococcum, Micrococcus album, Micrococcus sulfurous, Desulfovibrio vulgaris,
Acinetobacter sp; Rhodococcus eruthropolis, Rhodococcus luteus, Rhodococcus
terrae, Basillus sp. isolated from samples of oil field pipelines.
The optimal
conditions for the synthesis of AAA: equimolar ratio of the starting materials;
temperature -5- 0 oC, the solvent THF, the reaction time of 4 hours.
In this case it has been
determined that very maximum yield of product is I
synthesized AAA – I- 89,6; II- 85,0; III- 75,2; IV- 71,0; V- 68,7 and VI-
81.2%.
Literature:
1. Medvedova A.C., Novokshonov V.V., Demina M.M. Voronkov
M.G. // Journal Organometall. Chem. 1998. Vol. 553. ¹1.
pp. 481-486.
2. Nesmeyanov A.N., Nesmeyanov N.A. Beginning of the
organic chemistry. M.: 1969. Vol.1. 381 p.
3. Agronomov A.E. Selected chapters of organic chemistry.
M.: Chemistry, 1990. pp. 264-287.
4. Temnikova T.I. The course of theoretical bases of
organic chemistry. L. 1962. pp. 414-423.
5. Temkin O.N. Acetylene chemistry "acetylene
tree" in organic chemistry XXI century // Soros Educational Journal. 2001.
T.7. ¹6. pp. 32-41.
6. Mavlanov M.E., Nurmanov S.E., Ziyadullaev O.E. The
bacterial flora of oil fields Kukdumalak and North Urtabulak // Uzbek Oil and
Gas Journal. 2013. ¹2. pp. 73-78.
7. Ziyadullaev O.E., Mirhamitova D.H., Nurmanov S.E.
Synthesis of 1-Phenyl-3,4-dimethylpentyn-1-ol-3 and its vinyl ether // Journal
Reports of the Academy of Sciences of Uzbekistan. 2012. ¹3. pp. 40-45.
8. Shchelkunov S.A., Sivolobova A.O., Mataeva S.O.,
Minbaev D.B., Muldahmedov Z.M. Grinyara reacting the
2-methyl-4-chlorobut-3-yn-2-ol in environments substituting diethyl ether//
Journal of Organic Chemistry. 2001. ¹1. pp. 17-37.
9. Ziyadullaev O.E. Synthesis and
technological of aromatic acetylenic alcohols, their vinyl ethers on the base
of phenylacetylene: des. cand. chem.
sc. Tashkent. 2011. p. 213.
10. Ziyadullayev
O.E. Mahatova G.B. Synthesis of aromatic acetylenic
alcohols ORT Publishing-Àpplied Sciences in Europe: tendencies of contemporary
development, Stuttgart, Germany, 2014. pp. 415-417.