Ѕ»ќЋќгические
науки/5.ћолекул€рна€
биологи€
Dvorshchenko
K.O., Dranitsina A.S., Ostapchenko L.I.
Taras Shevchenko Kyiv National
University, Kyiv, Ukraine
Expression
of Gast and ChgA genes in rat
liver during long-term gastric
hypochlorhydria and its correction with administration of multiprobiotic
Long-term gastric hypoacidity is accompanied by disturbance of the digestive processes, dysbiosis, hypergastrinemia, and is one of the risk factor of gastric tumors [1]. Long-term inhibition of gastric acid secretion can causes pathological changes in liver cells. Firstly, gastric hypochlorhydria reduces the stimulating effect of cholecystokinin and secretin (which are excreted to the duodenum under the influence of hydrochloric acid) on bile secretion. Secondly,
†bacterial infections with reduced
gastric acidity can be cause of metabolic liver diseases. According to a scientific
literature, expression of Gast and ChgA genes is associated with development of
neuroendocrine tumors, including liver [2, 3].
Formerly we have shown that multiprobiotic "Symbiter![]() acidophilic" concentrated
(УSymbiterФ) prevented morphological changes in liver, evoked by prolonged hypoacidity. Multiprobiotic
УSymbiterФ Ц the
concentrated biomass of living cells of multicomponent symbiotic probiotic
bacteria (Bifidobacterium, Lactobacillus, lactic streptococci and propionic
acid bacteria).
acidophilic" concentrated
(УSymbiterФ) prevented morphological changes in liver, evoked by prolonged hypoacidity. Multiprobiotic
УSymbiterФ Ц the
concentrated biomass of living cells of multicomponent symbiotic probiotic
bacteria (Bifidobacterium, Lactobacillus, lactic streptococci and propionic
acid bacteria).
The aim of the study was to
determine the expression of Gast and ChgA genes in rat liver in conditions of
long-term hypoacidity, evoked by omeprazole and the effect of
multiprobiotic УSymbiterФ on this process.
Materials and
methods. Experiments were carried out on white non-strain male rats with initial
weight around 180-200 g. All animals were divided into four groups. Rats
injected abdominally with 0,2 ml of physiological solution and 0,5 ml of water
for injections orally were used as a control (first group). Animals of second
group were treated with the same dose of УSymbiterФ orally (0,14 ml/kg) during 28 days. Hypoacidity (third
group) was modeled by everyday intraperitoneal injection of omeprazole (14
mg/kg) during 28 days. Fourth experimental group simultaneously with omeprazole
obtained УSymbiterФ orally (0,14 ml/kg). Number
of animals in each experimental group was 7.
Total RNAs from rat liver were
isolated accordingly to modification of the procedure of Chomczynski et al.
(1987). The expression of above mentioned genes was analyzed by
semiquantitative RT-PCR. Statistical
processing of experimental data was performed with analysis of varience.
Probability of difference between control and test measurements was assessed
with StudentТs t-test.
Results
and discussion. mRNA of Gast and ChgA genes was not detected in rat liver
of control group and the group of rats injected with УSymbiterФ (Fig. 1, 2).
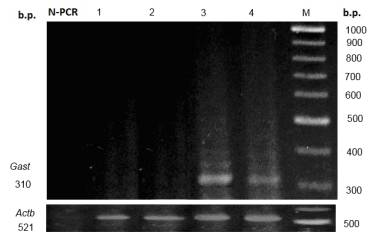
Fig. 1. Level of gastrin mRNA in rat liver upon long-term gastric hypochlorhydria
and with administration of multiprobiotic УSymbiterФ.
ћ Ц molecular mass marker; 1 Ц control; 2 Ц Symbiter; 3 Ц omeprazole; 4 Ц omeprazole + Symbiter; N-PCR Ц negative PCR control.
At the same time, upon
long-term gastric hypoacidity expression of this genes was observed, since its
mRNA was revealed in the samples of rat livers. It was
shown that prolonged inhibition of gastric secretion of hydrochloric acid by
omeprazole caused appearance of
Gast and ChgA genes expression in liver (Fig. 1, 2).
At combined
introduction of animal УSymbiterФ with omeprazole,
the expression of Gast and ChgA genes decreased by 3.1 and
1.2 times accordingly relative to the group of rats injected with omeprazole (Fig. 1, 2).
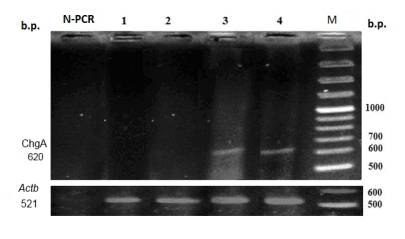
Fig. 2. Level of chromogranin A mRNA in rat liver upon long-term gastric hypochlorhydria
and with administration of multiprobiotic УSymbiterФ.
ћ Ц molecular mass marker; 1 Ц control; 2 Ц Symbiter; 3 Ц omeprazole; 4 Ц omeprazole + Symbiter; N-PCR Ц negative PCR control.
The long-term hypoacidity of gastric juice is accompanied with the changes in expression of Gast and ChgA genes in the liver tissue that indicates the potential risk of cell transformation. Symbiter promoted
†partial restoration of the investigated genes in liver via normalization microbiota in the
gastrointestinal tract and diminishing of serum gastrin level.
Literature.
1. Canani R., Terrin G. Gastric acidity inhibitors and
the risk of intestinal infections // Curr. Opin. Gastroenterol. Ц
2010. Ц
Vol. 26, є1. Ц P. 31-35.
2. Harris J., Gilliam A., McKenzie A. et al. The
biological and therapeutic importance of gastrin gene expression in pancreatic
adenocarcinomas // Cancer Res. Ц 2004. Ц Vol. 64. Ц P. 5624-5631.
3. Massironi S., Fraquelli M., Paggi S. et. al. Chromogranin A
levels in chronic liver disease and hepatocellular carcinoma // Dig. Liver. Dis. Ц 2009. Ц
Vol. 41, є1. Ц P. 31-35.