Isolation
of the alcohol oxidase from the methylotrophic yeast cells genera Pichia, Hansenula
Zaytcev M.
Tula State University
For
trace aliphatic alcohols determination are mainly used gas and HPLC methods, spectrophotometry
and refractometry methods. However despite the low limits of detection of
alcohols, general shortcomings of these methods are the complexity of the
equipment, in some cases - complexity sample preparation and the duration
analysis. At the same time enzymatic methods are successfully used for alcohol
determine with combine high sensitivity and selectivity with the simplicity of
the equipment and processing methods, rapidity and efficiency. Enzyme sensors
for ethanol determination may be developed which immobilized enzyme alcohol
oxidase (AO). The yeast Pichia pastories,
Pichia angusta, Hansenula polymorpha are source of the AO. Development of a
technique for isolation and purification of the alcohol oxidase from
methylotrophic yeasts with modular system for the separation and purification
of proteins which can be used for large-scale production of biological
preparations based modern bioanalytical and economical automated equipment is
the actual problem.
The
methylotrophic yeast P. angusta VKM
Y-2518, P. angusta VKM Y-1397, P. angusta VKM Y-2559, H. polymorpha NCYC 495 ln are used as a
source of alcohol oxidase. The method of enzyme isolation included the
following steps: yeast cells breaking with ultrasonic disrupter, a stepwise
proteins precipitation with ammonium sulphate, ion-exchange chromatography on
DEAE sepharose with semi-preparative proteins separation system Biologic LP
(BIO-RAD), alcohol oxidase concentration. Enzyme purification was monitored at
all stages with polyacrylamide gel electrophoresis.
The
cells were disrupted with an ultrasonic disintegrator; insoluble cell debris
was separated by centrifugation (8000g). The protein fraction was stepwise
fractionated ammonium sulfate in a concentration range of ammonium sulfate
saturation of 20-100%. Specific activity of enzyme preparations and protein
content were determined in the supernatant and the precipitate at all stages of
salting. Figure 1 shows the total amount of protein and specific activity of
the enzyme preparation at each stage of salting out.
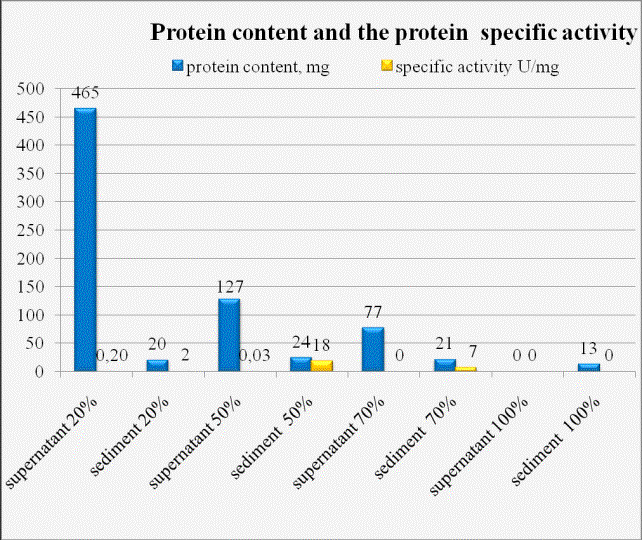
Figure 1: Total
protein content and specific activity at different stages AO salting. AO (H. polymorpha NCYC 495ln).
Highest
specific activity of AO corresponds to the precipitated proteins fraction at a
concentration of 50% ammonium sulfate saturation and 70% ammonium sulfate saturation.
A similar distribution was observed to AO for all methylotrophic yeast strains.
The fractions with the highest specific activity of AO were combined and used
in the final purification step.
Further
proteins purification from outside AO performed using ion exchange
chromatography. Alcohol oxidase desorption was performed phosphate buffer
solution containing potassium chloride (1M), with linearly increasing
percentage of KCl in the final solution (0-100%). As the alcohol oxidase
molecular weight is a 600kDa collected enzyme can be further concentrated using
with concentrating filter (100KDa) on the centrifuge (8000g). The concentrated
enzyme is a light - yellow color substance. The specific activity and the
protein content of the enzyme alcohol oxidase preparations isolated from
various yeast strains are shown in Table 1
Table
1. The specific activity and the protein content for the enzyme alcohol oxidase
preparations derived from various methylotrophic yeast strains
|
№ |
alcohol oxidase producer
strain |
total protein content, mg |
Specific enzyme activity
U/mg of protein |
|
1 |
P. angusta ВКМ Y-2518 |
- |
- |
|
2 |
P. angusta ВКМ Y-2559 |
10 |
9 |
|
3 |
P. angusta ВКМ Y-1397 |
9 |
16 |
|
4 |
H. polymorpha NCYC 495 ln |
11 |
19 |
Thus
the alcohol oxidase specific activity isolated from Hansenula polymorpha NCYC
495 ln higher then specific activity of AO isolated from other studied strains.
The high protein content and high activity of the isolated enzyme showed the
promising use of this strain for the production of the alcohol oxidase.
The
work was supported by the Federal Goal-oriented Program “Scientific and
Scientific-Pedagogical Cadres of Innovative Russia” for 2009–2013, agreement No
14.B37.21.0561 and State contract No 16.740.11.0766