The substrate specificity of
the membrane fraction of bacteria G.
oxydans in conditions of the biofuel cell
Minaycheva P.R., Kirillov I.M., Alferov S.V.
Tula State University
Bacteria of Gluconobacter sp. as well as Acetobacter sp. are long known in biotechnology
for their unique oxidative metabolism. Gluconobacter cells
contain multiple membrane-bound dehydrogenase.
Oxidation of
monosaccharide and alcohol is carried out either in the pentose phosphate cycle
in the cytosol by cytoplasmic, preferably NADH-dependent enzymes, or partial
oxidation reactions surfactant localized dehydrogenase. It is known that the
acetic acid bacteria contain membrane-bond glucose and alcohol dehydrogenase.
Individual enzymes and whole cells of microorganisms are competing types of
biocatalysts for use in the biofuel cell (BFC). Membrane localization of Gluconobacter oxydans enzyme allows use
a membrane fraction of the biocatalyst that can be an alternative to the use of
enzymes. Firstly, it is more economically advantageous since the process of
obtaining a membrane fraction is an intermediate step in isolation of
individual enzymes, and therefore less expensive. Second, the is the priority use of a wide range of
substrates that can be achieved using a membrane fraction containing various
membrane-bond dehydrogenase.
Enzyme
preparations containing membrane-bond dehydrogenase G. oxydans, prepared by ultrasonically disrupted of the bacterial
biomass by means of sequential centrifugation. From the literature about using
membrane fractions of bacteria G. oxydans
mediator in biosensors. Given the similarity of structures mediator graphite
paste electrode with the electrodes used in the BFC, we can talk about the
possibility of using membrane fractions of bacteria G. oxydans as a biocatalyst in the layout of the biofuel cell.
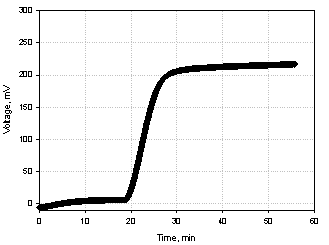
A typical plot of the potential generated from the BFC model based on membrane fraction of G. oxydans is shown in figure 1. Potential
measurements have been carried out in the sodium phosphate buffer solution
using galvanicpotentiostatic analyzer IPC Micro. Graphite rods have been dipped
into electrochemical cell. The registration of the potential generation while
substrate was added to the anode chamber has been carried out after stationary
potential range achievement. The measurable parameter during the biocatalytic
substrate oxidation was the potential signal amplitude. Evaluation of the catalytic
activity of the bacterial enzyme systems were performed in the closed external
circuit mode.
Fig.1 Typical plot of the potential generated from the BFC
model based on membrane fraction of G. oxydans
It is
known that bacteria Gluconobacter typical
substrates are carbohydrates and alcohols, most of these compounds are oxidized
by oxygen and membrane-bond dehydrogenase associated with bacterial respiratory
chain. This process is also the basis for recording the oxidative activity of
enzyme systems using bacterial synthetic electron acceptor (2,6-dichlorophenol
indophenol).
The absolute values of
the potentials generated in the layout of the BFC using the membrane fraction
of G. oxydans are shown in figure 2.
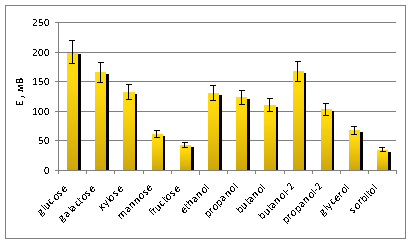
Fig. 2 Absolute
values of the
potentials generated in the layout of the BFC using the membrane fraction of G.
oxydans
It was
found that the maximum potential generated observed with glucose (200 ± 10 mV),
galactose (160 ± 10 mV), and butanol-2 (170 ± 10 mV) as substrates.
Thus,
the relative magnitude of the potential difference in response to galactose was
made 83% other monosaccharides -
from 22 to 70%. In response to the relative values of the generated alcohols capacity lie within the
range from 35 to 84% compared to glucose.
Previously,
the layout of the biofuel cell based on a suspension of G. oxydans values were obtained power
characteristics. Comparative evaluation of the power characteristics for BFC
models based on a variety of biocatalysts is shown in table 1. Glucose was used
as substrate (concentration of 10 mM).
Òable 1
Power characteristics of the BFC models with biocatalysts based on G. oxydans
|
Type of biocatalyst |
Voltage, mV |
External resistance, kΩ |
Internal resistance, kΩ |
Power, ŋW |
Power density, µW·m-2 |
|
Supension of bacterial cells
|
120±10 |
240 |
300 |
52±3 |
7 |
|
Membrane fraction |
200±10 |
100 |
120 |
160±10 |
20 |
The use
of biocatalysts based on the membrane fraction can double the voltage compare
with the suspension of bacterial cells, and the 2,5 times lower internal
resistance and to increase power density three times. Thus, membrane fraction
of bacteria G. oxydans as a
biocatalyst is promising to significantly increase the energy characteristics
of the layout biofuel cell.
The work was supported by government assignment
¹ 4412 and President Grant for young Ph.D. ¹ 16.120.11.4341-MK.